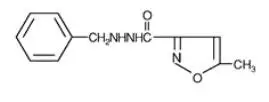
Drug Detail:Marplan (Isocarboxazid [ eye-so-kar-box-a-zid ])
Drug Class: Monoamine oxidase inhibitors
Suicidality and Antidepressant Drugs
Antidepressants increased the risk compared to placebo of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults in short-term studies with Major Depressive Disorder (MDD) and other psychiatric disorders. Anyone considering the use of Marplan or any other antidepressant in a child, adolescent or young adult must balance this risk with the clinical need. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction in risk with antidepressants compared to placebo in adults aged 65 and older. Depression and certain other psychiatric disorders are themselves associated with increases in the risk of suicide. Patients of all ages who are started on antidepressant therapy should be monitored appropriately and observed closely for clinical worsening, suicidality, or unusual changes in behavior. Families and caregivers should be advised of the need for close observation and communication with the prescriber. Marplan is not approved for use in pediatric patients (see Warnings: Clinical Worsening and Suicide Risk, Precautions: Information for Patients, and Precautions: Pediatric Use).
Pooled analyses of short-term (4 to 16 weeks) placebo-controlled trials of 9 antidepressant drugs (SSRIs and others) in children and adolescents with major depressive disorder (MDD), obsessive compulsive disorder (OCD), or other psychiatric disorders (a total of 24 trials involving over 4400 patients) have revealed a greater risk of adverse events representing suicidal thinking or behavior (suicidality) during the first few months of treatment in those receiving antidepressants. The average risk of such events in patients receiving antidepressants was 4%, twice the placebo risk of 2%. No suicides occurred in these trials.
Contraindications
Marplan (isocarboxazid) should not be administered in combination with any of the following: MAO inhibitors or dibenzazepine derivatives; sympathomimetics (including amphetamines); some central nervous system depressants (including narcotics and alcohol); antihypertensive, diuretic, antihistaminic, sedative or anesthetic drugs, buproprion HCL, buspirone HCL, dextromethorphan, cheese or other foods with a high tyramine content; or excessive quantities of caffeine.
Marplan (isocarboxazid) should not be administered to any patient with a confirmed or suspected cerebrovascular defect or to any patient with cardiovascular disease, hypertension, or history of headache.
Contraindicated Patient Populations
Hypersensitivity
Marplan should not be used in patients with known hypersensitivity to isocarboxazid.
Cerebrovascular Disorders
Marplan should not be administered to any patient with a confirmed or suspected cerebrovascular defect or to any patient with cardiovascular disease or hypertension.
Pheochromocytoma
Marplan should not be used in the presence of pheochromocytoma, as such tumors secrete pressor substances whose metabolism may be inhibited by Marplan.
Liver Disease
Marplan should not be used in patients with a history of liver disease, or in those with abnormal liver function tests.
Renal Impairment
Marplan should not be used in patients with severe impairment of renal function.
Contraindicated MAOI-Other Drug Combinations
Other MAOI Inhibitors or With Dibenzazepine-Related Entities
Marplan should not be administered together with, or in close proximity to, other MAO inhibitors or dibenzazepine-related entities. Hypertensive crises, severe convulsive seizures, coma, or circulatory collapse may occur in patients receiving such combinations.
In patients being transferred to Marplan from another MAO inhibitor or from a dibenzazepine-related entity, a medication-free interval of at least 1 week should be allowed, after which Marplan therapy should be started using half the normal starting dosage for at least the first week of therapy. Similarly, at least 1 week should elapse between the discontinuation of Marplan and initiation of another MAO inhibitor or dibenzazepine-related entity, or the readministration of Marplan. The following list includes some other MAO inhibitors, dibenzazepine-related entities, and tricyclic antidepressants.
| Generic Name | Trademark (Manufacturer) |
| Other MAO Inhibitors | |
| Furazolidone | Furoxone® (Roberts Laboratories) |
| Pargyline HCL | Eutonyl® (Abbott Laboratories) |
| Pargyline HCL and methyclothiazide | Eutron® (Abbott Laboratories) |
| Phenelzine sulfate | Nardil® (Parke-Davis) |
| Procarbazine | Matulane® (Roche Laboratories) |
| Tranylcypromine sulfate | Parnate® (SmithKline Beecham Pharmaceuticals) |
| Dibenzazepine-Related and Other Tricyclics | |
| Amitriptyline HCL | Elavil® (Zeneca) |
| Endep® (Roche Products) | |
| Perphenazine and amitriptyline HCL | Etrafon® (Schering) |
| Triavil® (Merck Sharp & Dohme) | |
| Clomipramine hydrochloride | Anafranil® (Novartis) |
| Desipramine HCL | Norpramin® (Hoechst Marion Roussel) |
| Pertofrane® (Rhône-Poulenc Rorer Pharmaceuticals) | |
| Imipramine HCL | Janimine® (Abbott Laboratories) |
| Tofranil® (Novartis) | |
| Nortriptyline HCL | Aventyl® (Eli Lilly & Co.) |
| Pamelor® (Novartis) | |
| Protripyline HCL | Vivactil® (Merck Sharp & Dohme) |
| Doxepin HCL | Adapin® (Fisons) |
| Sinequan® (Pfizer) | |
| Carbamazepine | Tegretol® (Novartis) |
| Cyclobenzaprine HCL | Flexeril® (Merck Sharp & Dohme) |
| Amoxapine | Asendin® (Lederle) |
| Maprotiline HCL | Ludiomil® (Novartis) |
| Trimipramine maleate | Surmontil® (Wyeth-Ayerst Laboratories) |
Warnings
Clinical Worsening and Suicide Risk
Patients with major depressive disorder (MDD), both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled trials of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled trials of nine antidepressant drugs (SSRIs) and others) in children and adolescents with MDD, Obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term trials of 9 antidepressant drugs in over 4400 patients. The pooled analyses of placebo-controlled trials in adults with MDD or other psychiatric disorders included 295 short-term trials (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk among drugs, but a tendency toward an increase in the younger patients `for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1000 patients treated) are provided in Table 1.
| Table 1 | |
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality Per 1000 Patients Treated |
| Increases Compared to Placebo | |
| <18 | 14 additional cases |
| 18 to 24 | 5 additional cases |
| Decreases Compared to Placebo | |
| 25 to 64 | 1 fewer case |
| >65 | 6 fewer cases |
No suicides occurred in any of the pediatric trials. There were suicides in the adult trials, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a casual link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset or were not part of the patient’s presenting symptoms.
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to health care providers. Such monitoring should include daily observation by families and caregivers. Prescriptions for MARPLAN should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
Precautions
Information for Patients
Prescribers or other health professionals should inform patients, their families, and their caregivers about the benefits and risks associated with treatment with Marplan and should counsel them in its appropriate use. A patient Medication Guide about “Antidepressant Medications, Depression and Other Serious Mental Illness, and Suicidal Thoughts and Actions” is available for Marplan. The prescriber or health professional should instruct patients, their families, and their caregivers to read the Medication Guide and should assist them in understanding its contents. Patients should be given the opportunity to discuss the contents of the Medication Guide and to obtain answers to any questions they may have. The complete text of the Medication Guide is reprinted at the end of this document.
Patients should be advised of the following issues and asked to alert their prescriber if these occur while taking Marplan.
| MARPLAN
isocarboxazid tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Validus Pharmaceuticals LLC (801194619) |