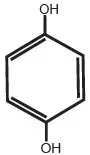
Drug Detail:Melquin hp (Hydroquinone topical [ hye-droe-kwin-one-top-ik-al ])
Drug Class: Topical depigmenting agents
Warnings
1. Hydroquinone is a skin bleaching agent which may produce undesired effects if not used as directed. The physician should be familiar with the contents of this insert before prescribing or dispensing this medication.
2. To evaluate possible susceptibility to irritation or sensitivity, each patient should begin by applying the medication to a small portion of unbroken skin at or near the pigmented area over a period of several days. Minor redness is not necessarily a contraindication, but treatment should be discontinued if itching, excessive inflammation or vesicle formation occurs. Close patient supervision is recommended. Use of MELQUIN® HP 4% CREAM in paranasal and infraorbital areas increases the chance of irritations (See ADVERSE REACTIONS). If no improvement is seen after two months of treatment, use of this product should be discontinued.
3. There are no sunscreens in MELQUIN® HP 4% CREAM. Sunscreen use is an essential aspect of hydroquinone therapy since even minimal sunlight exposure stimulates melanocyte activity. During the depigmentation maintenance treatment subsequent to the intensive depigmentation therapy, sun exposure of the bleached skin should be avoided to prevent repigmentation. For daytime treatment, use NUQUIN® HP 4% CREAM, NUQUIN® HP 4% GEL or MELPAQUE® HP 4% CREAM.
4. Contains Sodium Metabisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
5. Keep this and all other medications out of reach of children. In case of accidental ingestion, call a physician or a poison control center immediately.
6. Avoid contact with eyes. In case of accidental contact, patient should rinse eyes thoroughly with water and contact physician. A bitter taste and antiseptic effect may occur if applied to the lips.
Adverse Reactions/Side Effects
No systemic adverse reactions have been reported. Occasional hypersensitivity (localized contact dermatitis) may occur in which case the medication should be discontinued and the physician notified immediately.
| MELQUIN HP
hydroquinone cream |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Stratus Pharmaceuticals (789001641) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Sonar Products, Inc. | 104283945 | MANUFACTURE(58980-472) | |