Drug Detail:Mepsevii (Vestronidase alfa [ ves-tron-i-dase-al-fa ])
Drug Class: Lysosomal enzymes
Highlights of Prescribing Information
MEPSEVII® (vestronidase alfa-vjbk) injection, for intravenous use
Initial U.S. Approval: 2017
WARNING: ANAPHYLAXIS
See full prescribing information for complete boxed warning.
-
Anaphylaxis has occurred with MEPSEVII administration, as early as the first dose (5.1), therefore appropriate medical support should be readily available when MEPSEVII is administered.
-
Closely observe patients during and for 60 minutes after MEPSEVII infusion (2.2, 5.1).
- Immediately discontinue the MEPSEVII infusion if the patient experiences anaphylaxis (2.2, 5.1).
Indications and Usage for Mepsevii
MEPSEVII is a recombinant human lysosomal beta glucuronidase indicated in pediatric and adult patients for the treatment of Mucopolysaccharidosis VII (MPS VII, Sly syndrome).
Limitations of Use
The effect of MEPSEVII on the central nervous system manifestations of MPS VII has not been determined. ( 1)
Mepsevii Dosage and Administration
- The recommended dosage is 4 mg/kg administered every two weeks as an intravenous infusion. (
2.1)
- Premedication with a non-sedating antihistamine with or without an anti-pyretic is recommended 30 to 60 minutes prior to the start of the infusion. (
2.2,
5.1)
- Administer the infusion over approximately 4 hours. In the first hour of infusion, infuse 2.5% of the total volume. After the first hour, the rate can be increased to infuse the remainder of the volume over 3 hours as tolerated. See Table 1 in the full prescribing information for the rate of infusion by dose and body weight. (
2.4)
- For additional information on preparation, administration, and storage see the full prescribing information. ( 2.3, 2.4)
Dosage Forms and Strengths
Injection: 10 mg/5 mL (2 mg/mL) in a single-dose vial ( 3)
Contraindications
None ( 4)
Adverse Reactions/Side Effects
Most common adverse reactions (≥1 patient) are: Infusion site extravasation, diarrhea, rash, anaphylaxis, infusion site swelling, peripheral swelling and pruritus. ( 6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Ultragenyx at 1-888-756-8657 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 12/2020
Full Prescribing Information
1. Indications and Usage for Mepsevii
MEPSEVII is indicated in pediatric and adult patients for the treatment of Mucopolysaccharidosis VII (MPS VII, Sly syndrome).
Limitations of Use
The effect of MEPSEVII on the central nervous system manifestations of MPS VII has not been determined.
2. Mepsevii Dosage and Administration
2.1 Recommended Dosage
MEPSEVII should be administered under the supervision of a healthcare professional with the capability to manage anaphylaxis. Premedication is recommended 30 to 60 minutes prior to the start of the infusion [see Dosage and Administration (2.2)].
The recommended dosage of MEPSEVII is 4 mg/kg administered by intravenous infusion every two weeks.
Administer the infusion over approximately 4 hours. Infuse the first 2.5% of the total volume over the first hour. After the first hour, increase the infusion rate as tolerated in order to complete infusion over the following 3 hours according to the recommended rate guidelines in Table 1 [see Dosage and Administration (2.4)].
2.2 Premedication
- Administration of a non-sedating antihistamine with or without an anti-pyretic medication is recommended 30 to 60 minutes prior to the start of the infusion for patient comfort.
- Follow the instructions in Table 1 for the rate of MEPSEVII infusion
[see Dosage and Administration (2.4)].
- Observe patients closely during the infusion and following the infusion for a minimum of 60 minutes for the development of anaphylaxis
[see Warnings and Precautions (5.1)].
- Discontinue the infusion immediately if the patient experiences a severe systemic reaction, including anaphylaxis [see Warnings and Precautions (5.1)].
2.3 Preparation Instructions
Prepare MEPSEVII according to the following steps using aseptic technique:
1. Determine the number of vials to be diluted based on the patient’s actual weight and the recommended dose of 4 mg/kg, using the following calculations (a-b):
a. Total dose (mg) = Patient’s weight (kg) x 4 mg/kg (recommended dose)
b. Total number of vials = Total dose (mg) divided by 10 mg/vial
2. Round to the next whole vial and remove the required number of vials from the refrigerator to allow them to reach room temperature. Do not heat, microwave or shake vials.
a. Volume (mL) of calculated dose = Total dose (mg) divided by the 2 mg/mL concentration
3. The final solution will be a 1:1 dilution of MEPSEVII with 0.9% Sodium Chloride Injection, USP. More than 1:1 dilution may be used if the patient can tolerate additional infusion volume, taking into consideration cardiac function and fluid status.
4. For a 1:1 dilution, prepare the solution at room temperature, as follows:
a. Select an empty infusion bag, sized upon the total volume of the final solution.
b. Prior to withdrawing MEPSEVII from the vial, visually inspect the solution for particulate matter and discoloration. Because this is a protein solution, slight flocculation (thin translucent fibers) may occur. The MEPSEVII solution should be colorless to slightly yellow. Discard if the solution is discolored or if there is particulate matter in the solution.
c. Slowly withdraw the volume of the calculated MEPSEVII dose from the appropriate number of vials (step 2a) using caution to avoid excessive agitation and any air or frothing. Use a sufficiently large needle (18 gauge) to minimize bubbles in the solution.
d. Slowly add MEPSEVII to the infusion bag using care to avoid agitation, ensuring liquid to liquid contact without generating bubbles or turbulence.
e. Add 0.9% Sodium Chloride Injection, USP equal to the volume of MEPSEVII to the infusion bag.
f. Gently rock the infusion bag to ensure proper distribution of MEPSEVII. Do not shake the solution.
2.4 Administration Instructions
Administer MEPSEVII as follows:
- The rate of infusion: In the first hour infuse 2.5% of the total volume, and infuse the remaining volume over the subsequent three hours (see Table 1).
Account for any dead space in the lines to ensure 2.5% of the total infusion volume is delivered into the patient’s bloodstream during the first hour of infusion.
- Use an infusion set equipped with an in-line, low-protein binding 0.2 micron filter to administer the diluted MEPSEVII solution.
- Do not flush the line containing MEPSEVII to avoid a rapid bolus of infused enzyme. Due to the low infusion rate, additional saline may be added through a separate line (piggyback or Y tube) to maintain sufficient intravenous flow to prevent clotting or line blockage.
- Do not infuse with other products in the infusion tubing. Compatibility with other products has not been evaluated.
- Use MEPSEVII immediately after dilution and complete the infusion within 42 hours from the time of dilution. Discard any unused product.
Stability
If immediate use is not possible, the diluted solution may be stored up to 36 hours under refrigeration at 2°C to 8°C (36°F to 46°F) followed by up to 6 hours at room temperature up to a maximum of 25°C (77°F).
| Patient
Weight Range (kg) | Total MEPSEVII
Dose Range (mg) | Total MEPSEVII Volume (rounded)
(mL) | Total
Infusion Volume of Drug and diluent (infused over 4 hours) (mL) | Infusion Rate for 1st Hour (2.5%) (mL/h) | Infusion Rate per Hour for Subsequent 3 Hours (97.5%/3)
(mL/h) |
| 3.5-5.9 | 14-23.6 | 10 | 20 | 0.5 | 6.5 |
| 6-8.4 | 24-33.6 | 15 | 30 | 0.8 | 9.8 |
| 8.5-10.9 | 34-43.6 | 20 | 40 | 1 | 13 |
| 11-13.4 | 44-53.6 | 25 | 50 | 1.3 | 16.3 |
| 13.5-15.9 | 54-63.6 | 30 | 60 | 1.5 | 19.5 |
| 16-18.4 | 64-73.6 | 35 | 70 | 1.8 | 22.8 |
| 18.5-20.9 | 74-83.6 | 40 | 80 | 2 | 26 |
| 21-23.4 | 84-93.6 | 45 | 90 | 2.3 | 29.3 |
| 23.5-25.9 | 94-103.6 | 50 | 100 | 2.5 | 32.5 |
| 26-28.4 | 104-113.6 | 55 | 110 | 2.8 | 35.8 |
| 28.5-30.9 | 114-123.6 | 60 | 120 | 3 | 39 |
| 31-33.4 | 124-133.6 | 65 | 130 | 3.3 | 42.3 |
| 33.5-35.9 | 134-143.6 | 70 | 140 | 3.5 | 45.5 |
| 36-38.4 | 144-153.6 | 75 | 150 | 3.8 | 48.8 |
| 38.5-40.9 | 154-163.6 | 80 | 160 | 4 | 52 |
| 41-43.4 | 164-173.6 | 85 | 170 | 4.3 | 55.3 |
| 43.5-45.9 | 174-183.6 | 90 | 180 | 4.5 | 58.5 |
| 46-48.4 | 184-193.6 | 95 | 190 | 4.8 | 61.8 |
| 48.5-50.9 | 194-203.6 | 100 | 200 | 5 | 65 |
| 51-53.4 | 204-213.6 | 105 | 210 | 5.3 | 68.3 |
| 53.5-55.9 | 214-223.6 | 110 | 220 | 5.5 | 71.5 |
| 56-58.4 | 224-233.6 | 115 | 230 | 5.8 | 74.8 |
| 58.5-60.9 | 234-243.6 | 120 | 240 | 6 | 78 |
| 61-63.4 | 244-253.6 | 125 | 250 | 6.3 | 81.3 |
| 63.5-65.9 | 254-263.6 | 130 | 260 | 6.5 | 84.5 |
| 66-68.4 | 264-273.6 | 135 | 270 | 6.8 | 87.8 |
| 68.5-70.9 | 274-283.6 | 140 | 280 | 7 | 91 |
3. Dosage Forms and Strengths
Injection: 10 mg/5 mL (2 mg/mL) as a colorless to slightly yellow liquid in a single-dose vial.
5. Warnings and Precautions
5.1 Anaphylaxis
Anaphylaxis to MEPSEVII was reported in 2 of 20 patients in the clinical program [see Adverse Reactions (6.1)]. These reactions occurred during MEPSEVII infusion and were observed as early as the first dose of MEPSEVII for one patient. Manifestations included respiratory distress, cyanosis, decreased oxygen saturation, and hypotension. The two patients with anaphylaxis to MEPSEVII during the clinical trials had one occurrence each and tolerated subsequent infusions of MEPSEVII, without recurrence.
Anaphylaxis can be life-threatening. MEPSEVII should be administered under the supervision of a healthcare professional with the capability to manage anaphylaxis. Patients should be observed for 60 minutes after MEPSEVII administration. If severe systemic reactions occur, including anaphylaxis, immediately discontinue the MEPSEVII infusion and provide appropriate medical treatment. Prior to discharge, inform patients of the signs and symptoms of anaphylaxis and instruct them to seek immediate medical care if symptoms occur. Consider the risks and benefits of re-administering MEPSEVII following anaphylaxis.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described below and elsewhere in the labeling:
- Anaphylaxis [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The MEPSEVII clinical program included 23 patients aged 5 months to 25 years who received treatment with MEPSEVII at doses up to 4 mg/kg once every two weeks for up to 187 weeks. Nineteen patients were younger than 18 years of age.
Table 2 summarizes the adverse reactions that occurred in Study 301, a randomized start trial in 12 patients with MPS VII between the ages of 8 and 25 years [see Clinical Studies (14)].
Adverse reactions in Table 2 occurred in one or more patients treated with MEPSEVII at a dosage of 4 mg/kg at a higher patient frequency than placebo. Adverse reaction incidence rates are presented in the table below to account for the different duration of exposure to active treatment vs. placebo.
| Adverse Reaction | MEPSEVII
N =12 n (Incidence Rate*) | Placebo
N=9 n (Incidence Rate*) |
| Infusion site extravasation | 4 (0.5) | 1 (0.4) |
| Diarrhea | 3 (0.4) | 0 (0.0) |
| Rash | 3 (0.4) | 2 (0.7) |
| Anaphylaxis | 2 (0.2) | 0 (0.0) |
| Infusion site swelling | 1 (0.1) | 0 (0.0) |
| Peripheral swelling | 1 (0.1) | 0 (0.0) |
| Pruritus | 1 (0.1) | 0 (0.0) |
n = number of reactions
*Adverse reaction incidence rates calculated per 8.3 patient years for exposure to MEPSEVII, and 2.7 years of exposure for placebo
Febrile Convulsion
One patient receiving a dose of 4 mg/kg experienced a febrile convulsion during MEPSEVII treatment at Week 66. The infusion was stopped, the patient received anticonvulsants, antipyretics and antibiotics, and the adverse reaction resolved. The patient subsequently was re-challenged without recurrence and continued on treatment.
14. Clinical Studies
The clinical program for MEPSEVII included 23 patients with MPS VII, 17 of whom were evaluable for efficacy, 20 for safety, and 23 for immunogenicity. Patients were enrolled in clinical trials and expanded access protocols receiving treatment at doses up to 4 mg/kg once every two weeks for up to 187 weeks. The patients ranged in age from 5 months to 25 years. Sixteen patients were younger than 18 years of age.
Studies 301 and 202
Study UX003-CL301 (referred to as Study 301, NCT02230566) was a randomized start trial of MEPSEVII 4 mg/kg every two weeks in patients with MPS VII. Twelve patients were randomized to one of four placebo durations before crossing over to active treatment. Three patients received MEPSEVII immediately for a duration of 48 weeks, 3 patients received placebo for 8 weeks then MEPSEVII for 40 weeks, 3 patients received placebo for 16 weeks then MEPSEVII for 32 weeks, and 3 patients received placebo for 24 weeks then MEPSEVII for 24 weeks. Of the 12 patients enrolled in the trial, 4 were male and 8 were female and ranged in age from 8 to 25 years (median 14 years). Nine patients were younger than 18 years of age. The majority of the patients were white (75%), with 50% of Hispanic or Latino ethnicity. Patients who were enrolled in Study 301 were eligible to roll over to Study UX003-CL202 (referred to as Study 202, NCT02432144), an open-label extension trial in which patients received additional doses of MEPSEVII at 4 mg/kg intravenously every other week for up to 144 weeks. Ten patients rolled over directly from the end of Study to Week 0 of Study 202, while 2 patients (17%) had treatment gaps before enrolling in Study 202.
In Study 301, motor function, forced vital capacity, and visual acuity were assessed after 24 weeks of MEPSEVII treatment and measured against pre-specified minimal important differences. The extremely small population of patients with MPS VII globally necessitated the enrollment of all patients able to participate resulting in a highly heterogeneous group. Clinical endpoints were not assessable in some patients due to their extent of disease, age or level of cognition. Repeated assessments of the six minute walk test (6MWT) were feasible in ten of 12 patients and are described further below. Of the three patients who improved on their 6MWT (Figure 1, left panel), two also were noted to have improvement in balance and gross motor proficiency as assessed by the Bruininks-Oseretsky Test of Motor Proficiency (BOT-2).
In this trial, the mean difference in 6MWT distance between MEPSEVII and placebo treatment periods in patients able to perform the test at baseline and subsequent visits through Week 24 is shown in Table 3. The mean difference in 6MWT distance increases with increased treatment duration, however, due to the small size of the trial, standard errors are large.
| Duration of MEPSEVII Treatment | LS mean 6MWT (meters) (± Standard Error)* | Number and Treatment Assignment of Patients Included in Analyis** |
| 8 weeks | -11 (± 24) | 5 placebo period; 8 MEPSEVII period |
| 16 weeks | 13 (± 32) | 5 placebo period; 8 MEPSEVII period |
| 24 weeks | 18 (± 33) | 5 placebo period; 8 MEPSEVII period |
*ANCOVA analysis of change from baseline in least squares (LS) mean between placebo and MEPSEVII for different periods, after adjusting for study cohort, age, and baseline 6MWT distance. Patients who used assistive devices were imputed as zeros in the analysis.
**Number and treatment assignment of patients included in the analysis was based upon a randomized start trial design and patient ability to complete testing. Due to no placebo period for the three patients who received 48 weeks of MEPSEVII in the first cohort of the randomized start design, more data were available for analyses during the treatment period (n=8) than during the placebo period (n=5). While data from 8 participants were available at each time point, due to missing observations, the 8 participants were not the same across all time points.
The observed individual 6MWT distances for the 10 patients who could perform the test in Study 301 and Study 202 through Week 184 are presented in Figure 1. The course of three patients with improvement in distance walked of at least 60 meters during the 301 Study compared to the start of MEPSEVII treatment (Week 0) is shown in the left panel; the relatively stable course in the remaining seven patients, including those who used assistive devices, is shown in the right panel.
Figure 1. 6MWT Distance for MPS VII Patients in Studies 301 and 202
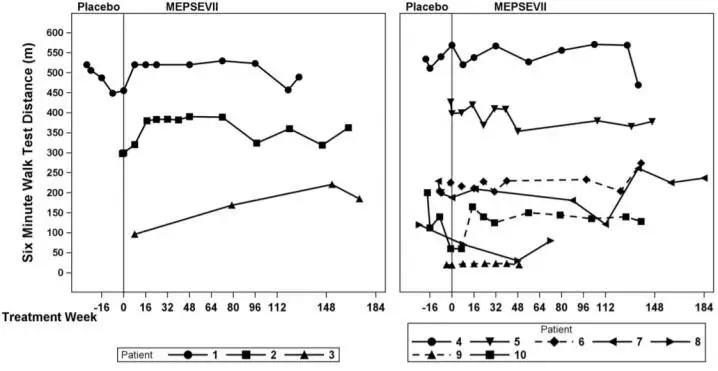
Patient 10 did not use an assistive device at baseline but started using an assistive device post-baseline from Treatment Week 8. Patients 6 and 9 consistently used an assistive device at all visits. A solid line indicates the unassisted assessments and a dotted line indicates the assisted assessments.
Liver and Spleen Volume
In Study 301, imaging by MRI or ultrasound to assess liver and spleen volume was performed in seven of the 12 patients. Most liver volumes were normal or below normal size at baseline (mean 1,591 mL, range 742 to 2,207 mL), and on average were unchanged after treatment (mean 1,459 mL, range 876 to 1,851 mL).
Spleen volumes generally were normal or below normal size at baseline (mean 325 mL, range 131 to 491 mL) and on average were unchanged after treatment (mean 360 mL, range 200 to 582 mL).
Study 203
UX003-CL203 (referred to as Study 203; NCT 02418455 ) was an open-label, uncontrolled single arm study that enrolled 8 patients less than 5 years of age who received MEPSEVII at a dose of 4 mg/kg every two weeks for 48 weeks of treatment and up to an additional 240 weeks during an optional continuation period. The study evaluated urinary GAG excretion, growth and hepatosplenomegaly. With long-term treatment, urinary GAG levels remained decreased upon exposure to MEPSEVII. At baseline, all 8 patients had impaired growth, and height remained near the 5th percentile relative to age-matched gender norms throughout the trial. No significant changes in hepatosplenomegaly were observed.
Other Investigations
Study UX003-CL201 (referred to as Study 201, NCT01856218) was a single arm, open-label, dose exploration trial completed outside the United States that enrolled three MPS VII patients, ranging in age from 5 years to 25 years. Two patients were male; two patients were white and one was Asian. After 120 weeks of exposure to MEPSEVII, one patient demonstrated a 21% improvement over baseline in forced vital capacity (FVC% predicted) on pulmonary function testing in addition to a 105 meter improvement in the 6MWT. Two other patients with baseline hepatosplenomegaly had reduction in liver volume (24% and 53%) and spleen volume (28% and 47%) after 36 weeks of MEPSEVII treatment.
Expanded access to MEPSEVII treatment was provided to a pediatric patient with MPS VII who required continuous ventilatory support at the start of treatment and was subsequently able to tolerate 9 hours daily off ventilator support after 164 weeks of MEPSEVII treatment.
| MEPSEVII
vestronidase alfa injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Ultragenyx Pharmaceutical Inc. (962892019) |