Drug Detail:Metronidazole (systemic) (monograph) (Flagyl)
Drug Class:
Metronidazole Description
Metronidazole tablets, 500 mg is an oral formulation of the synthetic nitroimidazole antimicrobial, 2-methyl-5-nitro-1H-imidazole-1-ethanol, which has the following structural formula:

Metronidazole USP is a white to pale yellow, crystalline powder. It is sparingly soluble in water and alcohol; slightly soluble in ether and chloroform; soluble in dilute hydrochloric acid.
Each metronidazole tablet, USP intended for oral administration contains 500 mg of metronidazole. In addition, each tablet contains the following inactive ingredients: colloidal silicon dioxide, hypromellose, low-substituted hydroxypropyl cellulose, microcrystalline cellulose and stearic acid. Additionally each tablet contains opadry II white 02F580003 which contains hypromellose, polyethylene glycol, talc and titanium dioxide.
Metronidazole - Clinical Pharmacology
Absorption
Plasma concentrations of metronidazole are proportional to the administered dose. Oral administration of 250 mg, 500 mg, or 2,000 mg produced peak plasma concentrations of 6 mcg/mL, 12 mcg/mL, and 40 mcg/mL, respectively. Studies reveal no significant bioavailability differences between males and females; however, because of weight differences, the resulting plasma levels in males are generally lower.
Metabolism/Excretion
Renal clearance of metronidazole is approximately 10 mL/min/1.73 m 2. The average elimination half-life of metronidazole in healthy subjects is eight hours.
Renal Impairment
Decreased renal function does not alter the single-dose pharmacokinetics of metronidazole.
Subjects with end-stage renal disease (ESRD; CL CR= 8.1±9.1 mL/min) and who received a single intravenous infusion of metronidazole 500 mg had no significant change in metronidazole pharmacokinetics but had 2-fold higher Cmax of hydroxy-metronidazole and 5-fold higher C max of metronidazole acetate, compared to healthy subjects with normal renal function (CL CR= 126±16 mL/min). Thus, on account of the potential accumulation of metronidazole metabolites in ESRD patients, monitoring for metronidazole associated adverse events is recommended (see PRECAUTIONS).
Microbiology
Metronidazole, a nitroimidazole, exerts antibacterial effects in an anaerobic environment against most obligate anaerobes. Once metronidazole enters the organism by passive diffusion and activated in the cytoplasm of susceptible anaerobic bacteria, it is reduced; this process includes intracellular electron transport proteins such as ferredoxin, transfer of an electron to the nitro group of the metronidazole, and formation of a short-lived nitroso free radical. Because of this alteration of the metronidazole molecule, a concentration gradient is created and maintained which promotes the drug's intracellular transport. The reduced form of metronidazole and free radicals can interact with DNA leading to inhibition of DNA synthesis and DNA degradation leading to death of the bacteria. The precise mechanism of action of metronidazole is unclear.
Resistance
A potential for development of resistance exists against metronidazole.
Resistance may be due to multiple mechanisms that include decreased uptake of the drug, altered reduction efficiency, overexpression of the efflux pumps, inactivation of the drug, and/or increased DNA damage repair.
Metronidazole does not possess any clinically relevant activity against facultative anaerobes or obligate aerobes.
Antimicrobial Activity
Metronidazole has been shown to be active against most isolates of the following bacteria both in vitro and in clinical infections as described in the INDICATIONS AND USAGE section.
Gram-positive anaerobes
Clostridium species
Eubacterium species
Peptococcus species
Peptostreptococcus species
Gram-negative anaerobes
Bacteroides fragilis group ( B. fragilis, B. distasonis, B. ovatus, B. thetaiotaomicron, B.vulgatus)
Fusobacterium species
Protozoal parasites
Entamoeba histolytica
Trichomonas vaginalis
The following in vitro data are available, but their clinical significance is unknown:
Metronidazole exhibits in vitro minimal inhibitory concentrations (MIC's) of 8 mcg/mL or less against most (≥ 90%) isolates of the following bacteria; however, the safety and effectiveness of metronidazole in treating clinical infections due to these bacteria have not been established in adequate and well-controlled clinical trials.
Gram-negative anaerobes
Bacteroides fragilis group ( B. caccae, B. uniformis)
Prevotella species ( P. bivia, P. buccae, P. disiens)
Susceptibility Tests:
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
Related/similar drugs
prednisone, omeprazole, amoxicillin, doxycycline, pantoprazole, ciprofloxacin, cephalexinIndications and Usage for Metronidazole
Asymptomatic Trichomoniasis. Metronidazole tablets, USP are indicated in the treatment of asymptomatic T. vaginalis infection in females when the organism is associated with endocervicitis, cervicitis, or cervical erosion. Since there is evidence that presence of the trichomonad can interfere with accurate assessment of abnormal cytological smears, additional smears should be performed after eradication of the parasite.
Treatment of Asymptomatic Sexual Partners. T. vaginalis infection is a venereal disease. Therefore, asymptomatic sexual partners of treated patients should be treated simultaneously if the organism has been found to be present, in order to prevent reinfection of the partner. The decision as to whether to treat an asymptomatic male partner who has a negative culture or one for whom no culture has been attempted is an individual one. In making this decision, it should be noted that there is evidence that a woman may become reinfected if her sexual partner is not treated. Also, since there can be considerable difficulty in isolating the organism from the asymptomatic male carrier, negative smears and cultures cannot be relied upon in this regard. In any event, the sexual partner should be treated with metronidazole tablets in cases of reinfection.
Amebiasis. Metronidazole tablets, USP are indicated in the treatment of acute intestinal amebiasis (amebic dysentery) and amebic liver abscess.
In amebic liver abscess, metronidazole tablets therapy does not obviate the need for aspiration or drainage of pus.
Anaerobic Bacterial Infections. Metronidazole tablets, USP are indicated in the treatment of serious infections caused by susceptible anaerobic bacteria. Indicated surgical procedures should be performed in conjunction with metronidazole tablets therapy. In a mixed aerobic and anaerobic infection, antimicrobials appropriate for the treatment of the aerobic infection should be used in addition to metronidazole tablets.
INTRA-ABDOMINAL INFECTIONS, including peritonitis, intra-abdominal abscess, and liver abscess, caused by Bacteroides species including the B. fragilis group ( B. fragilis, B. distasonis, B. ovatus, B. thetaiotaomicron, B. vulgatus), Clostridium species, Eubacterium species, Peptococcus species, and Peptostreptococcus species.
SKIN AND SKIN STRUCTURE INFECTIONS caused by Bacteroides species including the B. fragilis group, Clostridium species, Peptococcus species, Peptostreptococcus species, and Fusobacterium species.
GYNECOLOGIC INFECTIONS, including endometritis, endomyometritis, tubo-ovarian abscess, and postsurgical vaginal cuff infection, caused by Bacteroides species including the B. fragilis group, Clostridium species, Peptococcus species, Peptostreptococcus species, and Fusobacterium species.
BACTERIAL SEPTICEMIA caused by Bacteroides species including the B. fragilis group and Clostridium species.
BONE AND JOINT INFECTIONS, (as adjunctive therapy), caused by Bacteroides species including the B. fragilis group.
CENTRAL NERVOUS SYSTEM (CNS) INFECTIONS, including meningitis and brain abscess, caused by Bacteroides species including the B. fragilis group.
LOWER RESPIRATORY TRACT INFECTIONS, including pneumonia, empyema, and lung abscess, caused by Bacteroides species including the B. fragilis group.
ENDOCARDITIS caused by Bacteroides species including the B. fragilis group.
To reduce the development of drug-resistant bacteria and maintain the effectiveness of metronidazole tablets and other antibacterial drugs, metronidazole tablets should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy. In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
Contraindications
Hypersensitivity
In patients with trichomoniasis, Metronidazole Tablets is contraindicated during the first trimester of pregnancy (see PRECAUTIONS).
Warnings
Central and Peripheral Nervous System Effects
Encephalopathy has been reported in association with cerebellar toxicity characterized by ataxia, dizziness, and dysarthria. CNS lesions seen on MRI have been described in reports of encephalopathy. CNS symptoms are generally reversible within days to weeks upon discontinuation of metronidazole. CNS lesions seen on MRI have also been described as reversible.
Peripheral neuropathy, mainly of sensory type has been reported and is characterized by numbness or paresthesia of an extremity.
Convulsive seizures have been reported in patients treated with metronidazole.
Aseptic meningitis: Cases of aseptic meningitis have been reported with metronidazole. Symptoms can occur within hours of dose administration and generally resolve after metronidazole therapy is discontinued.
The appearance of abnormal neurologic signs and symptoms demands the prompt evaluation of the benefit/risk ratio of the continuation of therapy (see ADVERSE REACTIONS).
Precautions
General
Drug Interactions
Drugs that Induce CYP450 Enzymes
Drugs that Prolong the QT interval
QT prolongation has been reported, particularly when metronidazole was administered with drugs with the potential for prolonging the QT interval.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Pulmonary tumors have been observed in all six reported studies in the mouse, including one study in which the animals were dosed on an intermittent schedule (administration during every fourth week only). Malignant liver tumors were increased in male mice treated at approximately 1500 mg/m 2 (similar to the maximum recommended daily dose, based on body surface area comparisons). Malignant lymphomas and pulmonary neoplasms were also increased with lifetime feeding of the drug to mice. Mammary and hepatic tumors were increased among female rats administered oral metronidazole compared to concurrent controls. Two lifetime tumorigenicity studies in hamsters have been performed and reported to be negative.
Metronidazole has shown mutagenic activity in in vitro assay systems including the Ames test. Studies in mammals in vivo have failed to demonstrate a potential for genetic damage.
Metronidazole failed to produce any adverse effects on fertility or testicular function in male rats at doses up at 400 mg/kg/day (similar to the maximum recommended clinical dose, based on body surface area comparisons) for 28 days. However, rats treated at the same dose for 6 weeks or longer were infertile and showed severe degeneration of the seminiferous epithelium in the testes as well as marked decreases in testicular spermatid counts and epididymal sperm counts. Fertility was restored in most rats after an eight week, drug-free recovery period.
Pregnancy:
Teratogenic Effects:
Metronidazole crosses the placental barrier and its effects on the human fetal organogenesis are not known. Reproduction studies have been performed in rats, rabbits, and mice at doses similar to the maximum recommended human dose based on body surface area comparisons. There was no evidence of harm to the fetus due to metronidazole.
Adverse Reactions/Side Effects
The following reactions have been reported during treatment with metronidazole:
Central Nervous System: The most serious adverse reactions reported in patients treated with metronidazole have been convulsive seizures, encephalopathy, aseptic meningitis, optic and peripheral neuropathy, the latter characterized mainly by numbness or paresthesia of an extremity. Since persistent peripheral neuropathy has been reported in some patients receiving prolonged administration of metronidazole, patients should be specifically warned about these reactions and should be told to stop the drug and report immediately to their physicians if any neurologic symptoms occur. In addition, patients have reported headache, syncope, dizziness, vertigo, incoordination, ataxia, confusion, dysarthria, irritability, depression, weakness, and insomnia (see WARNINGS).
Gastrointestinal: The most common adverse reactions reported have been referable to the gastrointestinal tract, particularly nausea, sometimes accompanied by headache, anorexia, and occasionally vomiting; diarrhea; epigastric distress; and abdominal cramping and constipation.
Mouth: A sharp, unpleasant metallic taste is not unusual. Furry tongue, glossitis, and stomatitis have occurred; these may be associated with a sudden overgrowth of Candida which may occur during therapy.
Dermatologic: Erythematous rash and pruritus.
Hematopoietic: Reversible neutropenia (leukopenia); rarely, reversible thrombocytopenia.
Cardiovascular: QT prolongation has been reported, particularly when metronidazole was administered with drugs with the potential for prolonging the QT interval. Flattening of the T-wave may be seen in electrocardiographic tracings.
Hypersensitivity: Urticaria, erythematous rash, Stevens-Johnson Syndrome, toxic epidermal necrolysis, flushing, nasal congestion, dryness of the mouth (or vagina or vulva), and fever.
Renal: Dysuria, cystitis, polyuria, incontinence, and a sense of pelvic pressure. Instances of darkened urine have been reported by approximately one patient in 100,000. Although the pigment which is probably responsible for this phenomenon has not been positively identified, it is almost certainly a metabolite of metronidazole and seems to have no clinical significance.
Hepatic: Cases of severe irreversible hepatotoxicity/acute liver failure, including cases with fatal outcomes with very rapid onset after initiation of systemic use of metronidazole, have been reported in patients with Cockayne syndrome (latency from drug start to signs of liver failure as short as 2 days) (see CONTRAINDICATIONS).
Other: Proliferation of Candida in the vagina, dyspareunia, decrease of libido, proctitis, and fleeting joint pains sometimes resembling "serum sickness." Rare cases of pancreatitis, which generally abated on withdrawal of the drug, have been reported.
Patients with Crohn's disease are known to have an increased incidence of gastrointestinal and certain extraintestinal cancers. There have been some reports in the medical literature of breast and colon cancer in Crohn's disease patients who have been treated with metronidazole at high doses for extended periods of time. A cause and effect relationship has not been established. Crohn's disease is not an approved indication for metronidazole tablets.
To report SUSPECTED ADVERSE REACTIONS, contact Viona Pharmaceuticals Inc. at 1-888-304-5011 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Overdosage
Oral metronidazole has been studied as a radiation sensitizer in the treatment of malignant tumors. Neurotoxic effects, including seizures and peripheral neuropathy, have been reported after 5 to 7 days of doses of 6 to 10.4 g every other day.
Treatment of Overdosage: There is no specific antidote for metronidazole overdose; therefore, management of the patient should consist of symptomatic and supportive therapy.
Metronidazole Dosage and Administration
Trichomoniasis
One-day treatment − two grams of metronidazole tablets, given either as a single dose or in two divided doses of one gram each, given in the same day.
Seven-day course of treatment − 250 mg three times daily for seven consecutive days. There is some indication from controlled comparative studies that cure rates as determined by vaginal smears and signs and symptoms, may be higher after a seven-day course of treatment than after a one-day treatment regimen.
The dosage regimen should be individualized. Single-dose treatment can assure compliance, especially if administered under supervision, in those patients who cannot be relied on to continue the seven-day regimen. A seven-day course of treatment may minimize reinfection by protecting the patient long enough for the sexual contacts to obtain appropriate treatment. Further, some patients may tolerate one treatment regimen better than the other.
Pregnant patients should not be treated during the first trimester (see CONTRAINDICATIONS). In pregnant patients for whom alternative treatment has been inadequate, the one-day course of therapy should not be used, as it results in higher serum levels which can reach the fetal circulation (see PRECAUTIONS, Pregnancy).
When repeat courses of the drug are required, it is recommended that an interval of four to six weeks elapse between courses and that the presence of the trichomonad be reconfirmed by appropriate laboratory measures. Total and differential leukocyte counts should be made before and after re-treatment.
In the Male: Treatment should be individualized as it is for the female.
Amebiasis
For acute intestinal amebiasis (acute amebic dysentery): 750 mg orally three times daily for 5 to 10 days.
For amebic liver abscess: 500 mg or 750 mg orally three times daily for 5 to 10 days.
Pediatric patients: 35 to 50 mg/kg/24 hours, divided into three doses, orally for 10 days.
Anaerobic Bacterial Infections
The usual adult oral dosage is 7.5 mg/kg every six hours (approx. 500 mg for a 70-kg adult). A maximum of 4 g should not be exceeded during a 24-hour period.
The usual duration of therapy is 7 to 10 days; however, infections of the bone and joint, lower respiratory tract, and endocardium may require longer treatment.
How is Metronidazole supplied
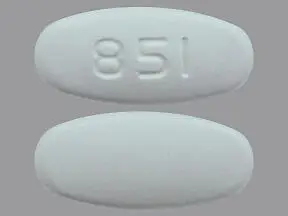
Metronidazole Tablets USP, 500 mg are white to off-white, oval shaped, biconvex, film coated tablets debossed with '851' on one side and plain on other side and are supplied as follows:
NDC 82982-039-14 in bottles of 14 tablets
Storage
Store at 20° to 25°C (68° to 77°F) [See USP Controlled Room Temperature] . Protect from light.
Dispense in a tight, light-resistant container (USP).
Manufacturedby:
Cadila Healthcare Ltd.,
India
Distributed by:
Viona Pharmaceuticals Inc.
Cranford, NJ 07016
Rev.: 12/21
Repackaged by:
Pharmasource Meds, LLC
Tigard, OR 97223
| METRONIDAZOLE
metronidazole tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
 |
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmasource Meds, LLC (118772692) |





