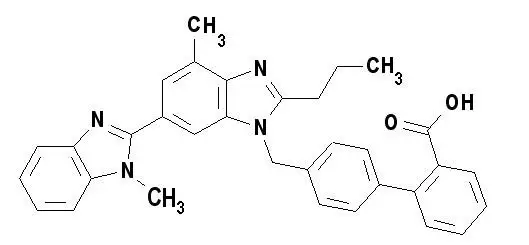
Drug Detail:Micardis (Telmisartan [ tel-mi-sar-tan ])
Drug Class: Angiotensin receptor blockers
Highlights of Prescribing Information
MICARDIS® (telmisartan tablets), for oral use
Initial U.S. Approval: 1998
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
- When pregnancy is detected, discontinue MICARDIS as soon as possible (5.1, 8.1)
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (5.1, 8.1)
Indications and Usage for Micardis
MICARDIS is an angiotensin II receptor blocker (ARB) indicated for:
- Treatment of hypertension, to lower blood pressure. Lowering blood pressure reduces the risk of fatal and nonfatal cardiovascular events, primarily strokes and myocardial infarctions. (1.1)
- Cardiovascular (CV) risk reduction in patients unable to take ACE inhibitors (1.2)
Micardis Dosage and Administration
- May be administered with or without food (2.1)
- When used for cardiovascular risk reduction, monitoring of blood pressure is recommended, and if appropriate, adjustment of medications that lower blood pressure may be necessary (2.2)
| Indication | Starting Dose | Dose Range |
| Hypertension (2.1) | 40 mg once daily | 40 to 80 mg once daily |
| Cardiovascular Risk Reduction (2.2) | 80 mg once daily | 80 mg once daily |
Dosage Forms and Strengths
- Tablets: 20 mg, 40 mg, 80 mg (3)
Contraindications
- Known hypersensitivity (e.g., anaphylaxis or angioedema) to telmisartan or any other component of this product (4)
- Do not co-administer aliskiren with MICARDIS in patients with diabetes (4)
Warnings and Precautions
- Avoid fetal or neonatal exposure (5.1)
- Hypotension: Correct any volume or salt depletion before initiating therapy. Observe for signs and symptoms of hypotension. (5.2)
- Monitor carefully in patients with impaired hepatic (5.4) or renal function (5.5)
- Avoid concomitant use of an ACE inhibitor and angiotensin receptor blocker (5.6)
Adverse Reactions/Side Effects
- Hypertension: The most common adverse events (≥1%) reported in hypertension trials are back pain, sinusitis, and diarrhea (6.1)
- Cardiovascular risk reduction: The serious adverse events (≥1%) reported in cardiovascular risk reduction trials were intermittent claudication and skin ulcer (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- NSAIDs: Increased risk of renal impairment and loss of anti-hypertensive effect (7)
- Do not co-administer aliskiren with MICARDIS in patients with diabetes (7)
Use In Specific Populations
- Lactation: Do not breastfeed during treatment with MICARDIS (8.2)
- Geriatric Patients: No overall difference in efficacy or safety vs younger patients, but greater sensitivity of some older individuals cannot be ruled out (8.5)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2022
Full Prescribing Information
WARNING: FETAL TOXICITY
- When pregnancy is detected, discontinue MICARDIS as soon as possible [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1) and Use in Specific Populations (8.1)].
1. Indications and Usage for Micardis
2. Micardis Dosage and Administration
3. Dosage Forms and Strengths
- 20 mg, white or off-white, round, uncoated tablets imprinted with BI logo on one side and "50H" on the other side
- 40 mg, white or off-white, oblong, uncoated tablets imprinted with BI logo on one side and "51H" on the other side
- 80 mg, white or off-white, oblong, uncoated tablets imprinted with BI logo on one side and "52H" on the other side
4. Contraindications
Do not co-administer aliskiren with MICARDIS in patients with diabetes [see Drug Interactions (7)].
5. Warnings and Precautions
6. Adverse Reactions/Side Effects
The following adverse reaction is described elsewhere in labeling:
- Renal dysfunction upon use with ramipril [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
| Telmisartan n=1455 % | Placebo n=380 % |
|
| Upper respiratory tract infection | 7 | 6 |
| Back pain | 3 | 1 |
| Sinusitis | 3 | 2 |
| Diarrhea | 3 | 2 |
| Pharyngitis | 1 | 0 |
6.2 Postmarketing Experience
Blood and Lymphatic System Disorders: Anemia, eosinophilia, thrombocytopenia
Gastrointestinal Disorders: Abdominal pain, diarrhea, dyspepsia, nausea
General Disorders and Administration Site Conditions: Asthenia, chest pain, edema, face edema, fatigue, lower limb edema, pain, weakness
Hepato-biliary: Abnormal hepatic function/liver disorder
Immune System Disorders: Anaphylactic reaction, hypersensitivity
Investigations: Increased CPK, uric acid increased
Metabolism and Nutrition Disorders: Hyperkalemia, hypoglycemia (in diabetic patients), hyponatremia
Musculoskeletal and Connective Tissue Disorders: Myalgia
Nervous System Disorders: Dizziness, headache, syncope
Renal and Urinary Disorders: Renal impairment including acute renal failure
Reproductive System and Breast Disorders: Erectile dysfunction
Respiratory, Thoracic and Mediastinal Disorders: Coughing
Skin and Subcutaneous Tissue Disorders: Angioedema (with fatal outcome), angioneurotic edema, drug eruption (toxic skin eruption mostly reported as toxicoderma, rash, and urticaria), erythema, sweating increased, urticaria
Vascular Disorder: Hypotension (including postural hypotension)
12. Micardis - Clinical Pharmacology
14. Clinical Studies
14.1 Hypertension
There were no changes in the heart rate of patients treated with telmisartan in controlled trials.
14.2 Cardiovascular Risk Reduction
| *The primary endpoint was defined as the time to first event. In case of multiple simultaneous events, all individual events were considered; the sum of patients with individual outcomes may exceed the number of patients with composite (primary or secondary) outcomes. **For individual components of the primary composite endpoints, all events, regardless whether or not they were the first event, were considered. Therefore, they are more than the first events considered for the primary or secondary composite endpoint. |
|||
| Telmisartan vs. Placebo (n=2954) (n=2972) |
|||
| No. of Events Telmisartan / Placebo | Hazard Ratio 95% CI | p-value | |
| *Composite of CV death, myocardial infarction, stroke, or hospitalization for heart failure | 465 (15.7%) / 504 (17.0%) | 0.92 (0.81 – 1.05) | 0.2129 |
| *Composite of CV death, myocardial infarction, or stroke | 384 (13.0%) / 440 (14.8%) | 0.87 (0.76 – 1.00) | 0.0483 |
| Individual components of the primary composite endpoint | No. of Events Telmisartan / Placebo | Hazard Ratio 95% CI | p-value |
| **All non-fatal MI | 114 (3.9%) / 145 (4.9%) | 0.79 (0.62 – 1.01) | 0.0574 |
| **All non-fatal strokes | 112 (3.8%) / 136 (4.6%) | 0.83 (0.64 – 1.06) | 0.1365 |
| Telmisartan vs. Ramipril (n=8542) (n=8576) |
||
| No. of Events Telmisartan / Ramipril | Hazard Ratio 97.5% CI |
|
| Composite of CV death, myocardial infarction, stroke, or hospitalization for heart failure | 1423 (16.7%) / 1412 (16.5%) | 1.01 (0.93 – 1.10) |
| Composite of CV death, myocardial infarction, or stroke | 1190 (13.9%) / 1210 (14.1%) | 0.99 (0.90 – 1.08) |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information)
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International GmbH, Ingelheim, Germany
Copyright © 2022 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
Boehringer Ingelheim Pharmaceuticals, Inc. either owns or uses the ONTARGET® and TRANSCEND™ trademarks under license.
The other brands listed are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc.
MICARDIS® (my-CAR-dis)
(telmisartan tablets)
What is the most important information I should know about MICARDIS tablets?
MICARDIS is a prescription medicine used:
- to treat high blood pressure (hypertension)
- in certain high risk people aged 55 years and older to help lower their risk of having certain cardiovascular problems such as stroke, heart attack, or death
It is not known if MICARDIS is safe and effective in children.
For patients with diabetes, if you are taking MICARDIS you should not take aliskiren.
What should I tell my doctor before taking MICARDIS tablets?
Before you take MICARDIS tablets, tell your doctor if you:
- are pregnant or are planning to become pregnant. See "What is the most important information I should know about MICARDIS tablets?"
- are breast-feeding or plan to breast-feed. It is not known if MICARDIS passes into your breast milk. You and your doctor should decide if you will take MICARDIS tablets or breast-feed. You should not do both. Talk with your doctor about the best way to feed your baby if you take MICARDIS tablets.
- have liver problems
- have kidney problems
- have heart problems
- have any other medical conditions
For patients with diabetes, if you are taking MICARDIS you should not take aliskiren.
- aliskiren
- digoxin (Lanoxin®)
- lithium (Lithobid®, lithium carbonate, lithium citrate)
- aspirin or other non-steroidal anti-inflammatory drugs (NSAIDs)
- other medicines used to treat your high blood pressure or heart problem
- water pills (diuretic)
How should I take MICARDIS tablets?
- Take MICARDIS tablets exactly as your doctor tells you to take it.
- Your doctor will tell you how much MICARDIS to take and when to take it.
- Do not change your dose unless your doctor tells you to.
- Take MICARDIS one time each day at the same time.
- Take MICARDIS tablets with or without food.
- If you miss a dose, take it as soon as you remember. If it is close to your next dose, do not take the missed dose. Take the next dose at your regular time.
- If you take too much MICARDIS, call your doctor, or go to the nearest hospital emergency room right away.
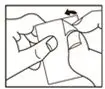
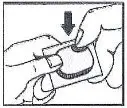
- Read the "How to Open the Blister" at the end of this leaflet before you use MICARDIS. Talk with your doctor if you do not understand the instructions.
What are the possible side effects of MICARDIS tablets?
MICARDIS tablets may cause serious side effects, including:
- Injury or death to your unborn baby. See "What is the most important information I should know about MICARDIS tablets?"
-
Low blood pressure (hypotension) is most likely to happen if you also:
- take water pills (diuretics)
- are on a low-salt diet
- get dialysis treatments
- have heart problems
- get sick with vomiting or diarrhea
- If you feel faint or dizzy, lie down and call your doctor right away.
-
Kidney problems, which may get worse if you already have kidney disease. You may have changes in your kidney test results, and you may need a lower dose of MICARDIS tablets. Call your doctor if you get:
- swelling in your feet, ankles, or hands
- unexplained weight gain
- Call your doctor right away if you get any of the symptoms listed above.
- High potassium in the blood (hyperkalemia). Your doctor may check your potassium levels as needed.
- swelling of the face, tongue, throat
- difficulty breathing
- skin rash
The most common side effects of MICARDIS tablets include:
- sinus pain and congestion (sinusitis)
- back pain
- diarrhea
How should I store MICARDIS tablets?
- Store MICARDIS tablets at room temperature between 68°F to 77°F (20°C to 25°C).
- Do not remove MICARDIS tablets from blisters until right before you take them.
Keep MICARDIS tablets and all medicines out of the reach of children.
General information about MICARDIS tablets
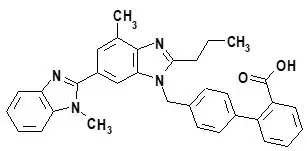
What are the ingredients in MICARDIS tablets?
Active Ingredient: telmisartan
Inactive Ingredients: sodium hydroxide, meglumine, povidone, sorbitol, and magnesium stearate
What is High Blood Pressure (Hypertension)?
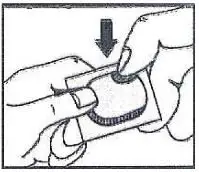
1. Tear (You may also use scissors to tear the blister apart)
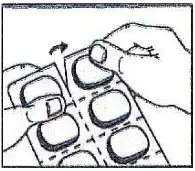
2. Peel (Peel off the paper layer from the aluminum foil)

3. Push (Push the tablet through the aluminum foil)
This Patient Information has been approved by the U.S. Food and Drug Administration
Distributed by:
Boehringer Ingelheim Pharmaceuticals, Inc.
Ridgefield, CT 06877 USA
Licensed from:
Boehringer Ingelheim International GmbH, Ingelheim, Germany
Copyright © 2022 Boehringer Ingelheim International GmbH
ALL RIGHTS RESERVED
Boehringer Ingelheim Pharmaceuticals, Inc. either owns or uses the ONTARGET® and TRANSCEND™ trademarks under license.
The other brands listed are trademarks of their respective owners and are not trademarks of Boehringer Ingelheim Pharmaceuticals, Inc.
| MICARDIS
telmisartan tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| MICARDIS
telmisartan tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| MICARDIS
telmisartan tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Promeco S.A de C.V. | 812579472 | MANUFACTURE(0597-0039, 0597-0040, 0597-0041) , PACK(0597-0039, 0597-0040, 0597-0041) , LABEL(0597-0039, 0597-0040, 0597-0041) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 551147440 | MANUFACTURE(0597-0039, 0597-0040, 0597-0041) , API MANUFACTURE(0597-0039, 0597-0040, 0597-0041) | |