Drug Detail:Mononine (Coagulation factor ix [ koe-ag-yoo-lay-shun-fak-tor-nine ])
Drug Class: Miscellaneous coagulation modifiers
Mononine Description
Coagulation Factor IX (Human), MONONINE is a sterile, stable, lyophilized concentrate of Factor IX prepared from pooled human plasma and is intended for use in therapy of Factor IX deficiency, known as Hemophilia B or Christmas disease. MONONINE is purified of extraneous plasma-derived proteins, including Factors II, VII and X, by use of immunoaffinity chromatography. A murine monoclonal antibody to Factor IX is used as an affinity ligand to isolate Factor IX from the source material. Factor IX is then dissociated from the monoclonal antibody, recovered, purified further, formulated and provided as a sterile, lyophilized powder. The immunoaffinity protocol utilized results in a highly pure Factor IX preparation. It shows predominantly a single component by SDS polyacrylamide electrophoretic evaluation and has a specific activity of not less than 190 Factor IX units per mg total protein.
All Source Plasma used in the manufacture of this product was tested by FDA-licensed Nucleic Acid Testing (NAT) for HBV, HCV, and HIV-1 and found to be nonreactive (negative).
This concentrate has been processed by monoclonal antibody immunoaffinity chromatography during its manufacture, which has been shown to be capable of reducing the risk of viral transmission. Additionally, a chemical treatment protocol and nanofiltration used in its manufacture have also been shown to be capable of significant virus reductions. However, no procedure has been shown to be totally effective in removing the risk of viral infectivity from coagulation factor concentrates (see CLINICAL PHARMACOLOGY and WARNINGS).
MONONINE is a purified preparation of Factor IX. When stored as directed, it will maintain its labeled potency for the period indicated on the container label.
Each vial contains the labeled amount of Factor IX activity expressed in International Units (IU). One IU represents the activity of Factor IX present in 1 mL of normal, pooled plasma. When reconstituted as recommended, the resulting solution is a clear, colorless, isotonic preparation of neutral pH, containing approximately 100 times the Factor IX potency found in an equal volume of plasma. Each mL of the reconstituted concentrate contains approximately 100 IU of Factor IX and non-detectable levels of Factors II, VII and X (<0.0025 IU per Factor IX unit using standard coagulation assays). Each vial also contains histidine (approx. 10mM), sodium chloride (approx. 0.066M), mannitol (approx. 3%) and polysorbate 80 (approx. 0.0075%). Hydrochloric acid and/or sodium hydroxide may have been used to adjust pH. MONONINE also contains trace amounts (≤50 ng mouse protein/100 Factor IX activity units) of the murine monoclonal antibody used in its purification (see CLINICAL PHARMACOLOGY).
MONONINE is to be administered only intravenously.
Mononine - Clinical Pharmacology
Hemophilia B, or Christmas disease, is an X-linked recessively inherited disorder of blood coagulation characterized by insufficient or abnormal synthesis of the clotting protein Factor IX. Factor IX is a vitamin K-dependent coagulation factor which is synthesized in the liver. Factor IX is activated by Factor XIa in the intrinsic coagulation pathway. Activated Factor IX (IXa), in combination with Factor VIII:C, activates Factor X to Xa, resulting ultimately in the conversion of prothrombin to thrombin and the formation of a fibrin clot. The infusion of exogenous Factor IX to replace the deficiency present in Hemophilia B temporarily restores hemostasis. Depending upon the subject's level of biologically active Factor IX, clinical symptoms range from moderate skin bruising or excessive hemorrhage after trauma or surgery to spontaneous hemorrhage into joints, muscles or internal organs including the brain. Severe or recurring hemorrhages can produce death, organ dysfunction or orthopedic deformity.
Infusion of Factor IX Complex concentrates that contain varying but significant amounts of the other liver-dependent blood coagulation proteins (Factors II, VII and X) into subjects with Hemophilia B, results in Factor IX recoveries ranging from approximately 0.57-1.1 IU/dL rise per IU/kg body weight infused with plasma half-lives for Factor IX ranging from approximately 23 hours to 31 hours.1,2 Infusion of MONONINE into ten subjects with severe or moderate Hemophilia B has shown a mean recovery of 0.67 IU/dL rise per IU/kg body weight infused and a mean half-life of 22.6 hours.3 After six months of experience with repeated infusions performed on the nine subjects who remained in the study, it was shown that the half-life and recovery was maintained at a level comparable to that found with the initial infusion. The six-month data showed a mean recovery of 0.68 IU/dL rise per IU/kg body weight infused and a mean half-life of 25.3 hours.3 The data show no statistically significant differences between the initial and six-month values.
Two studies were conducted to provide MONONINE for treatment of hemophilia B subjects who required extensive Factor IX replacement for surgery, trauma, or spontaneous bleeding (73 unique subjects and eight subjects enrolled twice for a total of 81 subjects), as well as to evaluate the safety and efficacy of MONONINE. The overall mean recovery during treatment was determined to be 1.23 ± 0.42 IU/dL rise/IU/kg (K) (range = 0.59 to 2.92 K) among the 55 subjects included in recovery analyses in the one study and to be 1.12 ± 0.52 K (range = 0.61 to 2.08 K) among 10 subjects included in these analyses in the second study. Five (5/81, 6%) subjects reported adverse reactions across the two studies. In these studies, 100 doses of MONONINE were administered at what are considered high doses for a Factor IX concentrate, a range of 71 to 161 IU/kg to a total of 36 subjects. Sixty-seven (67) of these infusions were the subject of recovery analyses. Mean recovery tended to decrease as the dose of MONONINE increased: 1.09 ± 0.52 K at doses >75-95 IU/kg (n=38), 0.98 ± 0.45 K at doses >95-115 IU/kg (n=21), 0.70 ± 0.38 K at doses >115-135 IU/kg (n=2), 0.67 K at doses >135-155 IU/kg (n=1), and 0.73 ± 0.34 K at doses >155 IU/kg (n=5). Among the 36 subjects who received these high doses, only one (2.8%) reported an adverse experience with a possible relationship to MONONINE ("difficulty in concentrating"; subject recovered). In no subjects were thrombogenic complications observed or reported.4
The manufacturing procedure for MONONINE includes multiple processing steps that have been designed to reduce the risk of virus transmission. Validation studies of the monoclonal antibody (MAb) immunoaffinity chromatography/chemical treatment step and nanofiltration step used in the production of MONONINE document the virus reduction capacity of the processes employed. These studies were conducted using the relevant enveloped and non-enveloped viruses. The results of these virus validation studies utilizing a wide range of viruses with different physicochemical properties are summarized in Table 1 below:
| Manufacturing Step | Virus Reduction Factor (log10) | |||||
|---|---|---|---|---|---|---|
| Enveloped Viruses | Non-Enveloped Viruses | |||||
| HIV-1 | BVDV | PRV | WNV | HAV | Parvovirus* | |
| HIV, Human immunodeficiency virus type 1, model for HIV-1 and HIV-2 | ||||||
| BVDV, Bovine viral diarrhea virus, model for HCV | ||||||
| PRV, Pseudorabies virus, model for large enveloped DNA viruses, e.g. herpes | ||||||
| WNV, West Nile virus | ||||||
| HAV, Hepatitis A virus | ||||||
|
||||||
| MAb Chromatography | ≥5.8 | 5.8 | ≥8.3 | ≥6.5 | [3.3]† | 6.61 |
| Nanofiltration | ≥4.4‡ | ≥6.8‡ | ≥7.8‡ | ≥6.3‡ | ≥6.7‡ | ≥8.22,‡ |
| Cumulative Virus Reduction (log10) | ≥10.2 | ≥12.6 | ≥16.1 | ≥12.8 | ≥6.7 | ≥14.8 |
Precautions
Information for Patients
Patients should be informed of the early symptoms and signs of hypersensitivity reactions including hives, generalized urticaria, tightness of the chest, dyspnea, wheezing, faintness, hypotension, and anaphylaxis. Patients should be advised to discontinue use of the product and contact their physician and/or seek immediate emergency care, depending on the severity of the reaction, if these symptoms occur.
Some viruses such as hepatitis A are particularly difficult to remove or inactivate at this time. Although the overwhelming number of hepatitis A cases are community acquired, there have been reports of these infections associated with the use of some plasma-derived products. Therefore, physicians should be alert to the potential symptoms of hepatitis A infections and inform patients under their supervision receiving plasma-derived products to report potential symptoms promptly.
Evidence of hepatitis A may include several days to weeks of poor appetite, tiredness, and low-grade fever followed by nausea, vomiting and pain in the belly. Dark urine and a yellowed complexion are also common symptoms. Patients should be encouraged to consult their physicians if such symptoms occur.
Mononine Dosage and Administration
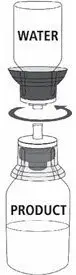
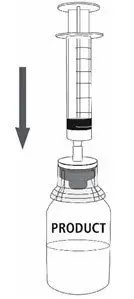
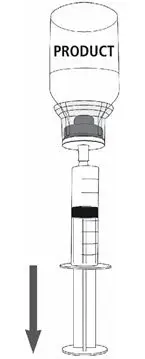
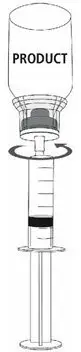
MONONINE is intended for intravenous administration only. It should be reconstituted with the volume of Sterile Water for Injection, USP supplied with the lot, and administered within three hours of reconstitution. Do not refrigerate after reconstitution. After administration, any unused solution and the administration equipment should be discarded.
As a general rule, 1 IU of Factor IX activity per kg can be expected to increase the circulating level of Factor IX by 1% [IU/dL] of normal. The following formula provides a guide to dosage calculations:
| Number of Factor IX | = | Body Weight | × | desired Factor IX | × | 1.0 IU/kg |
| IU required (IU) | (in kg) | increase (% or IU/dL normal) | [per IU/dL] | |||
The amount of MONONINE to be infused, as well as the frequency of infusions, will vary with each patient and with the clinical situation.11,12
As a general rule, the level of Factor IX required for treatment of different conditions is as follows:
| Minor Spontaneous Hemorrhage, Prophylaxis | Major Trauma or Surgery | |
|---|---|---|
| Desired levels of Factor IX for Hemostasis | 15-25% [or IU/dL] | 25-50% [or IU/dL] |
| Initial loading dose to achieve desired level | up to 20-30 IU/kg | up to 75 IU/kg |
| Frequency of dosing | once; repeated in 24 hours if necessary | every 18-30 hours, depending on T1/2 and measured Factor IX levels |
| Duration of treatment | once; repeated if necessary | up to ten days, depending upon nature of insult |
Recovery of the loading dose varies from patient to patient. Doses administered should be titrated to the patient's response. MONONINE administered in doses of ≥75 IU/kg were well tolerated (see CLINICAL PHARMACOLOGY).
In the presence of an inhibitor to Factor IX, higher doses of MONONINE might be necessary to overcome the inhibitor (see PRECAUTIONS). No data on the treatment of patients with inhibitors to Factor IX with MONONINE are available.
For information on rate of administration, see Rate of Administration, below.
Administration
Rate of Administration
The rate of administration should be determined by the response and comfort of the patient; intravenous dosage administration rates of up to 225 IU/minute have been regularly tolerated without incident. When reconstituted as directed, i.e., to approximately 100 IU/mL, MONONINE should be administered at a rate of approximately 2.0 mL per minute.
| MONONINE
coagulation factor ix human kit |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| MONONINE
coagulation factor ix human kit |
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
|
||||||||||||||||
| Labeler - CSL Behring LLC (058268293) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| CSL Behring LLC | 058268293 | MANUFACTURE(0053-6232, 0053-6233) | |