Drug Detail:Moxeza (Moxifloxacin ophthalmic [ mox-i-flox-a-sin ])
Drug Class: Ophthalmic anti-infectives
Highlights of Prescribing Information
MOXEZA® (moxifloxacin ophthalmic solution) 0.5%, for topical ophthalmic use
Initial U.S. Approval: 1999
Recent Major Changes
| Warnings and Precautions (5.1) | 8/2021 |
Indications and Usage for Moxeza
MOXEZA is a topical fluoroquinolone anti-infective indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms:
Aerococcus viridans*, Corynebacterium macginleyi*, Enterococcus faecalis*, Micrococcus luteus*, Staphylococcus arlettae*, Staphylococcus aureus, Staphylococcus capitis, Staphylococcus epidermidis, Staphylococcus haemolyticus, Staphylococcus hominis, Staphylococcus saprophyticus*, Staphylococcus warneri*, Streptococcus mitis*, Streptococcus pneumoniae, Streptococcus parasanguinis*, Escherichia coli*, Haemophilus influenzae, Klebsiella pneumoniae*, Propionibacterium acnes, Chlamydia trachomatis*
*Efficacy for this organism was studied in fewer than 10 infections. (1)
Moxeza Dosage and Administration
Instill 1 drop in the affected eye(s) 2 times daily for 7 days. (2)
Dosage Forms and Strengths
Ophthalmic solution containing moxifloxacin 0.5%. (3)
Contraindications
None. (4)
Warnings and Precautions
- Corneal Endothelial Damage and Toxic Anterior Segment Syndrome: Intracameral injections will cause harm to endothelium. (5.1)
- Hypersensitivity Reactions: Hypersensitivity and anaphylaxis have been reported with systemic use of moxifloxacin. (5.2)
- Prolonged Use: May result in overgrowth of non-susceptible organisms, including fungi. If superinfection occurs, discontinue use and institute alternative therapy. (5.3)
- Avoid Contact Lens Wear: Patients should not wear contact lenses if they have signs or symptoms of bacterial conjunctivitis. (5.4)
Adverse Reactions/Side Effects
The most common adverse reactions reported in 1% to 2% of patients were eye irritation, pyrexia, and conjunctivitis. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2021
Full Prescribing Information
1. Indications and Usage for Moxeza
MOXEZA is indicated for the treatment of bacterial conjunctivitis caused by susceptible strains of the following organisms:
Aerococcus viridans*
Corynebacterium macginleyi*
Enterococcus faecalis*
Micrococcus luteus*
Staphylococcus arlettae*
Staphylococcus aureus
Staphylococcus capitis
Staphylococcus epidermidis
Staphylococcus haemolyticus
Staphylococcus hominis
Staphylococcus saprophyticus*
Staphylococcus warneri*
Streptococcus mitis*
Streptococcus pneumoniae
Streptococcus parasanguinis*
Escherichia coli*
Haemophilus influenza
Klebsiella pneumoniae*
Propionibacterium acnes
Chlamydia trachomatis*
*Efficacy for this organism was studied in fewer than 10 infections.
5. Warnings and Precautions
5.1 Corneal Endothelial Damage and Toxic Anterior Segment Syndrome
NOT FOR INTRACAMERAL USE OR INJECTION. MOXEZA will cause damage to the corneal endothelium if introduced directly into the anterior chamber of the eye.
Toxic Anterior Segment Syndrome (TASS) has been reported following intraocular administration of moxifloxacin. TASS is typically characterized by anterior chamber inflammatory reactions, such as fibrin, cell or flare and corneal edema, but other events, such as hypopyon, keratic precipitates or vitreous opacities may also occur.
5.2 Hypersensitivity Reactions
In patients receiving systemically administered quinolones, including moxifloxacin, serious and occasionally fatal hypersensitivity (anaphylactic) reactions have been reported, some following the first dose. Some reactions were accompanied by cardiovascular collapse, loss of consciousness, angioedema (including laryngeal, pharyngeal or facial edema), airway obstruction, dyspnea, urticaria, and itching. If an allergic reaction to moxifloxacin occurs, discontinue use of the drug. Serious acute hypersensitivity reactions may require immediate emergency treatment. Oxygen and airway management should be administered as clinically indicated.
5.3 Growth of Resistant Organisms With Prolonged Use
As with other anti-infectives, prolonged use may result in overgrowth of non-susceptible organisms, including fungi. If superinfection occurs, discontinue use and institute alternative therapy. Whenever clinical judgment dictates, the patient should be examined with the aid of magnification, such as slit-lamp biomicroscopy, and, where appropriate, fluorescein staining.
6. Adverse Reactions/Side Effects
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to the rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to MOXEZA in 1263 patients, between 4 months and 92 years of age, with signs and symptoms of bacterial conjunctivitis. The most frequently reported adverse reactions were eye irritation, pyrexia and conjunctivitis, reported in 1% to 2% of patients.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no adequate and well-controlled studies with MOXEZA in pregnant women to inform any drug-associated risks.
Oral administration of moxifloxacin to pregnant rats and monkeys and intravenously to pregnant rabbits during the period of organogenesis did not produce adverse maternal or fetal effects at clinically relevant doses. Oral administration of moxifloxacin to pregnant rats during late gestation through lactation did not produce adverse maternal, fetal or neonatal effects at clinically relevant doses (see Data).
Data
Animal Data
Embryo-fetal studies were conducted in pregnant rats administered with 20, 100, or 500 mg/kg/day moxifloxacin by oral gavage on Gestation Days 6 to 17, to target the period of organogenesis. Decreased fetal body weight and delayed skeletal development were observed at 500 mg/kg/day (1420 times the human area under the curve (AUC) at the recommended human ophthalmic dose). The No-Observed-Adverse-Effect-Level (NOAEL) for developmental toxicity was 100 mg/kg/day (152 times the human AUC at the recommended human ophthalmic dose).
Embryo-fetal studies were conducted in pregnant rabbits administered with 2, 6.5, or 20 mg/kg/day moxifloxacin by intravenous administration on Gestation Days 6 to 20, to target the period of organogenesis. Abortions, increased incidence of fetal malformations, delayed fetal skeletal ossification, and reduced placental and fetal body weights were observed at 20 mg/kg/day (5569 times the human AUC at the recommended human ophthalmic dose), a dose that produced maternal body weight loss and death. The NOAEL for developmental toxicity was 6.5 mg/kg/day (1261 times the human AUC at the recommended human ophthalmic dose).
Pregnant cynomolgus monkeys were administered moxifloxacin at doses of 10, 30, or 100 mg/kg/day by intragastric intubation between Gestation Days 20 and 50, targeting the period of organogenesis. At the maternal toxic doses of ≥ 30 mg/kg/day, increased abortion, vomiting and diarrhea were observed. Smaller fetuses/reduced fetal body weights were observed at 100 mg/kg/day (14688 times the human AUC at the recommended human ophthalmic dose). The NOAEL for fetal toxicity was 10 mg/kg/day (894 times the human AUC at the recommended human ophthalmic dose).
In a pre- and postnatal study, rats were administered moxifloxacin by oral gavage at doses of 20, 100, and 500 mg/kg/day from Gestation Day 6 until the end of lactation. Maternal death occurred during gestation at 500 mg/kg/day. Slight increases in the duration of pregnancy, reduced pup birth weight, and decreased prenatal and neonatal survival were observed at 500 mg/kg/day (estimated 1420 times the human AUC at the recommended human ophthalmic dose). The NOAEL for pre- and postnatal development was 100 mg/kg/day (estimated 152 times the human AUC at the recommended human ophthalmic dose).
8.2 Lactation
Risk Summary
There are no data regarding the presence of MOXEZA in human milk, the effects on the breastfed infants, or the effects on milk production/excretion to inform risk of MOXEZA to an infant during lactation. A study in lactating rats has shown transfer of moxifloxacin into milk following oral administration. Systemic levels of moxifloxacin following topical ocular administration are low [see Clinical Pharmacology (12.3)], and it is not known whether measurable levels of moxifloxacin would be present in maternal milk following topical ocular administration. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for MOXEZA and any potential adverse effects on the breastfed child from MOXEZA.
8.4 Pediatric Use
The safety and effectiveness of MOXEZA in infants below 4 months of age have not been established.
There is no evidence that the ophthalmic administration of moxifloxacin has any effect on weight bearing joints, even though oral administration of some quinolones has been shown to cause arthropathy in immature animals.
11. Moxeza Description
MOXEZA is a sterile solution for topical ophthalmic use.
Moxifloxacin hydrochloride is an 8-methoxy fluoroquinolone anti-infective, with a diazabicyclononyl ring at the C7 position.
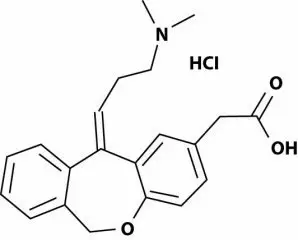
C21H24FN3O4•HCl Molecular Weight 437.9 g/mol
Chemical Name: 1-Cyclopropyl-6-fluoro-1,4-dihydro-8-methoxy-7-[(4aS,7aS)-octahydro-6H-pyrrolol[3,4-b]pyridin-6-yl]-4-oxo-3-quinolinecarboxylic acid, monohydrochloride.
Each mL of MOXEZA solution contains 5.45 mg moxifloxacin hydrochloride, equivalent to 5 mg moxifloxacin base.
Inactives: boric acid, hydrochloric acid and/or sodium hydroxide to adjust pH, purified water, sodium chloride, sorbitol, tyloxapol, and xanthan gum.
MOXEZA is a greenish-yellow, isotonic solution with an osmolality of 300-370 mOsm/kg and a pH of approximately 7.4. Moxifloxacin hydrochloride is a slightly yellow to yellow crystalline powder.
12. Moxeza - Clinical Pharmacology
12.1 Mechanism of Action
Moxifloxacin is a member of the fluoroquinolone class of anti-infective drugs [see Microbiology (12.4)].
12.3 Pharmacokinetics
Moxifloxacin steady-state plasma pharmacokinetics were evaluated in healthy adult male and female subjects who were administered multiple, bilateral, topical ocular doses of MOXEZA two times daily for four days with a final dose on Day 5. The average steady-state AUC0-12 was 8.17 ± 5.31 ng*h/mL. Moxifloxacin Cmax following twice-daily bilateral ophthalmic administration of moxifloxacin 0.5% for 5 days is approximately 0.02% of that achieved with the oral formulation of moxifloxacin hydrochloride (Cmax following oral dosing of 400 mg AVELOX*, 4.5 ± 0.5 mcg/mL).
12.4 Microbiology
The antibacterial action of moxifloxacin results from inhibition of the topoisomerase II (DNA gyrase) and topoisomerase IV. DNA gyrase is an essential enzyme that is involved in the replication, transcription and repair of bacterial DNA. Topoisomerase IV is an enzyme known to play a key role in the partitioning of the chromosomal DNA during bacterial cell division.
The mechanism of action for quinolones, including moxifloxacin, is different from that of macrolides, aminoglycosides, or tetracyclines. Therefore, moxifloxacin may be active against pathogens that are resistant to these antibiotics and these antibiotics may be active against pathogens that are resistant to moxifloxacin. There is no cross-resistance between moxifloxacin and the aforementioned classes of antibiotics. Cross-resistance has been observed between systemic moxifloxacin and some other quinolones.
In vitro resistance to moxifloxacin develops via multiple-step mutations. Resistance to moxifloxacin occurs in vitro at a general frequency of between 1.8 x 10-9 to < 1 x 10-11 for Gram-positive bacteria.
Moxifloxacin has been shown to be active against most strains of the following microorganisms, both in vitro and in clinical infections as described in the Indications and Usage section:
Aerococcus viridans*
Corynebacterium macginleyi*
Enterococcus faecalis*
Micrococcus luteus*
Staphylococcus arlettae*
Staphylococcus aureus
Staphylococcus capitis
Staphylococcus epidermidis
Staphylococcus haemolyticus
Staphylococcus hominis
Staphylococcus saprophyticus*
Staphylococcus warneri*
Streptococcus mitis*
Streptococcus pneumoniae
Streptococcus parasanguinis*
Escherichia coli*
Haemophilus influenza
Klebsiella pneumoniae*
Propionibacterium acnes
Chlamydia trachomatis*
*Efficacy for this organism was studied in fewer than 10 infections.
The following in vitro data are available, but their clinical significance in ophthalmic infections is unknown. The safety and effectiveness of MOXEZA in treating ophthalmic infections due to these organisms have not been established in adequate and well-controlled trials.
Moxifloxacin has been shown to be active in vitro against most strains of the microorganisms listed below. These organisms are considered susceptible when evaluated using systemic breakpoints; however, a correlation between the in vitro systemic breakpoint and ophthalmologic efficacy has not been established. The list of organisms is provided as guidance only in assessing the potential treatment of conjunctival infections. Moxifloxacin exhibits in vitro minimal inhibitory concentrations (MICs) of 2 mcg/mL or less (systemic susceptible breakpoint) against most (≥ 90%) strains of the following ocular pathogens.
Aerobic Gram-positive microorganisms:
Staphylococcus caprae
Staphylococcus cohnii
Staphylococcus lugdunensis
Staphylococcus pasteuri
Streptococcus agalactiae
Streptococcus milleri group
Streptococcus oralis
Streptococcus pyogenes
Streptococcus salivarius
Streptococcus sanguis
Aerobic Gram-negative microorganisms:
Acinetobacter baumannii
Acinetobacter calcoaceticus
Acinetobacter junii
Enterobacter aerogenes
Enterobacter cloacae
Haemophilus parainfluenzae
Klebsiella oxytoca
Moraxella catarrhalis
Moraxella osloensis
Morganella morganii
Neisseria gonorrhoeae
Neisseria meningitides
Pantoea agglomerans
Proteus vulgaris
Pseudomonas stutzeri
Serratia liquefaciens
Serratia marcescens
Stenotrophomonas maltophilia
Anaerobic microorganisms:
Clostridium perfringens
Peptostreptococcus anaerobius
Peptostreptococcus magnus
Peptostreptococcus micros
Peptostreptococcus prevotii
Other microorganisms:
Mycobacterium tuberculosis
Mycobacterium avium
Mycobacterium kansasii
Mycobacterium marinum
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long-term studies in animals to determine the carcinogenic potential of moxifloxacin have not been performed. However, in an accelerated study with initiators and promoters, moxifloxacin was not carcinogenic in rats following up to 38 weeks of oral dosing at 500 mg/kg/day (4741 times the recommended daily human ophthalmic dose for a 60 kg person, based on body surface area).
Mutagenesis
Moxifloxacin was not mutagenic in four bacterial strains used in the Ames Salmonella reversion assay. As with other quinolones, the positive response observed with moxifloxacin in strain TA 102 using the same assay may be due to the inhibition of DNA gyrase. Moxifloxacin was not mutagenic in the CHO/HGPRT mammalian cell gene mutation assay. An equivocal result was obtained in the same assay when v79 cells were used. Moxifloxacin was clastogenic in the v79 chromosome aberration assay, but it did not induce unscheduled DNA synthesis in cultured rat hepatocytes. There was no evidence of genotoxicity in vivo in a micronucleus test or a dominant lethal test in mice.
Impairment of Fertility
Moxifloxacin had no effect on fertility in male and female rats at oral doses as high as 500 mg/kg/day, approximately 4741 times the highest recommended daily human ophthalmic dose. At 500 mg/kg/day orally there were slight effects on sperm morphology (head-tail separation) in male rats and on the estrous cycle in female rats.
14. Clinical Studies
In one randomized, double-masked, multicenter, vehicle-controlled clinical trial in which patients with bacterial conjunctivitis were dosed with MOXEZA 2 times a day, MOXEZA was superior to its vehicle for both clinical and microbiological outcomes. Clinical cure achieved on Day 4 was 63% (265/424) in MOXEZA treated patients, versus 51% (214/423) in vehicle-treated patients. Microbiologic success (eradication of baseline pathogens) was achieved on Day 4 in 75% (316/424) of MOXEZA-treated patients versus 56% (237/423) of vehicle treated patients. Microbiologic eradication does not always correlate with clinical outcome in anti-infective trials.
16. How is Moxeza supplied
MOXEZA 0.5%, is supplied as a sterile ophthalmic solution in a dispensing system consisting of a natural low density polyethylene bottle and dispensing plug and tan polypropylene closure. Tamper evidence is provided with a shrink band around the closure and neck area of the package.
3 mL in a 4 mL bottle…………………………………………………………………………………………NDC 0065-0006-03
Storage: Store at 2°C to 25°C (36°F to 77°F).
17. Patient Counseling Information
Avoid Contamination of the Product
Advise patients not to touch the dropper tip to any surface to avoid contaminating the contents.
Avoid Contact Lens Wear
Advise patients not to wear contact lenses if they have signs and symptoms of bacterial conjunctivitis.
Hypersensitivity Reactions
Systemically administered quinolones, including moxifloxacin, have been associated with hypersensitivity reactions, even following a single dose. Advise patients to discontinue use immediately and contact their physician at the first sign of a rash or allergic reaction [see Warnings and Precautions (5.2)].
*AVELOX is a registered trademark of Bayer.
Distributed by:
Novartis Pharmaceuticals Corporation
East Hanover, New Jersey 07936
© Novartis
T2021-115
| MOXEZA
moxifloxacin hydrochloride solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Alcon Laboratories, Inc. (008018525) |





