Drug Detail:Mulpleta (Lusutrombopag [ loo-soo-trom-boe-pag ])
Drug Class: Platelet-stimulating agents
Highlights of Prescribing Information
MULPLETA ® (lusutrombopag tablets) for oral use
Initial U.S. Approval: 2018
Indications and Usage for Mulpleta
MULPLETA is a thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure. (1)
Mulpleta Dosage and Administration
- Begin MULPLETA dosing 8-14 days prior to a scheduled procedure. (2.1)
- Patients should undergo their procedure 2-8 days after the last dose. (2.1)
- Recommended Dosage: 3 mg orally once daily with or without food for 7 days. (2.1)
Dosage Forms and Strengths
Tablet: 3 mg. ( 3)
Contraindications
- None.
Warnings and Precautions
- Thrombotic/Thromboembolic Complications: MULPLETA is a thrombopoietin (TPO) receptor agonist, and TPO receptor agonists have been associated with thrombotic and thromboembolic complications in patients with chronic liver disease. Monitor platelet counts and for thromboembolic events and institute treatment promptly. (5.1)
Adverse Reactions/Side Effects
The most common adverse reaction (≥3%): headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Shionogi Inc. at 1-800-849-9707 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Lactation: Breastfeeding is not recommended during treatment. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 4/2020
Related/similar drugs
Doptelet, prednisone, dexamethasone, Decadron, Deltasone, PromactaFull Prescribing Information
1. Indications and Usage for Mulpleta
MULPLETA is indicated for the treatment of thrombocytopenia in adult patients with chronic liver disease who are scheduled to undergo a procedure.
2. Mulpleta Dosage and Administration
2.1 Recommended Dosage
Begin MULPLETA dosing 8-14 days prior to a scheduled procedure.
Patients should undergo their procedure 2-8 days after the last dose.
The recommended dosage of MULPLETA is 3 mg taken orally once daily with or without food for 7 days. In the case of a missed dose of MULPLETA, patients should take the missed dose as soon as possible on the same day and return to the normal schedule the following day.
MULPLETA has been investigated only as a single 7-day once daily dosing regimen in clinical trials in patients with chronic liver disease [see Clinical Studies (14)]. MULPLETA should not be administered to patients with chronic liver disease in an attempt to normalize platelet counts.
3. Dosage Forms and Strengths
Tablets: 3 mg lusutrombopag as a light red, round, film-coated tablet debossed with the Shionogi trademark (  ) above the identifier code "551" on one side and with a "3" on the other side.
) above the identifier code "551" on one side and with a "3" on the other side.
5. Warnings and Precautions
5.1 Thrombotic/Thromboembolic Complications
MULPLETA is a thrombopoietin (TPO) receptor agonist, and TPO receptor agonists have been associated with thrombotic and thromboembolic complications in patients with chronic liver disease. Portal vein thrombosis has been reported in patients with chronic liver disease treated with TPO receptor agonists. Portal vein thrombosis was reported in 1% (2 of 171) of MULPLETA-treated patients and 1% (2 of 170) of placebo-treated patients in 3 randomized, double-blind trials and was identified post-procedure in protocol-specified imaging. The thromboses were not associated with a marked increase in platelet count.
Consider the potential increased thrombotic risk when administering MULPLETA to patients with known risk factors for thromboembolism, including genetic pro-thrombotic conditions (Factor V Leiden, Prothrombin 20210A, Antithrombin deficiency, or Protein C or S deficiency). In patients with ongoing or prior thrombosis or absence of hepatopetal blood flow, MULPLETA should only be used if the potential benefit to the patient justifies the potential risk.
MULPLETA should not be administered to patients with chronic liver disease in an attempt to normalize platelet counts.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in detail in other sections of the labeling:
- Thrombotic/Thromboembolic Complications [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of MULPLETA was evaluated in 3 randomized, double-blind, placebo-controlled trials, L-PLUS 1, L-PLUS 2, and M0626, in which patients with chronic liver disease and thrombocytopenia were treated with MULPLETA (N=171) or placebo (N=170) at a dose of 3 mg daily for up to 7 days prior to a scheduled procedure.
The majority of patients were males (59%), and median age was 61 years (range 19-88). The racial and ethnic distribution was White (50%), Asian (47%), Black (<1%), and Other (3%).
The most common adverse reactions (those occurring in at least 3%) in the MULPLETA-treated group across the pooled data from the three trials are summarized in table 1.
| Adverse Reaction* | MULPLETA 3 mg (N=171) % | Placebo (N=170) % |
|---|---|---|
|
||
| Headache | 5 | 4 |
The incidence of serious adverse events was 5% (9 of 171 patients) in the MULPLETA group and 7% (12 of 170 patients) in the placebo group. The most common serious adverse reaction reported with MULPLETA was portal vein thrombosis [see Warnings and Precautions (5.1)]. No adverse reactions resulted in discontinuation of MULPLETA.
8. Use In Specific Populations
8.5 Geriatric Use
Clinical studies of MULPLETA did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients.
10. Overdosage
No antidote for MULPLETA overdose is known.
In the event of overdose, platelet count may increase excessively and result in thrombotic or thromboembolic complications. Closely monitor the patient and platelet count. Treat thrombotic complications in accordance with standard of care.
Hemodialysis is not expected to enhance the elimination of MULPLETA because lusutrombopag is highly bound to protein in plasma [see Clinical Pharmacology (12.3)].
11. Mulpleta Description
MULPLETA (lusutrombopag), a thrombopoietin (TPO) receptor agonist, contains lusutrombopag as the active ingredient.
The chemical name for lusutrombopag is (2 E)-3-{2,6-Dichloro-4-[(4-{3-[(1 S)-1-(hexyloxy) ethyl]-2-methoxyphenyl}-1,3-thiazol-2-yl) carbamoyl]phenyl}-2-methylprop-2-enoic acid.
The structural formula is:
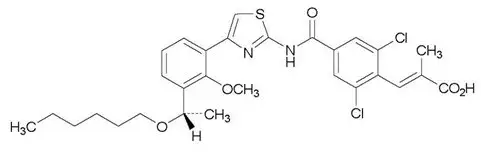
The empirical formula for lusutrombopag is C 29H 32Cl 2N 2O 5S and the molecular weight is 591.54.
Lusutrombopag is a white to slightly yellowish white powder, and is freely soluble in N,N-dimethylformamide, slightly soluble in ethanol (99.5%) and methanol, very slightly soluble in acetonitrile, and practically insoluble in water. Lusutrombopag is slightly soluble in the buffer solution at pH 11 and practically insoluble in buffer solutions with pH ranges of 1 to 9.
MULPLETA (lusutrombopag) tablets for oral use contain lusutrombopag 3 mg.
Excipients are D-mannitol, microcrystalline cellulose, magnesium oxide, sodium lauryl sulfate, hydroxypropyl cellulose, carboxymethylcellulose calcium, magnesium stearate, hypromellose, triethyl citrate, titanium dioxide, red ferric oxide, and talc.
12. Mulpleta - Clinical Pharmacology
12.1 Mechanism of Action
Lusutrombopag is an orally bioavailable, small molecule TPO receptor agonist that interacts with the transmembrane domain of human TPO receptors expressed on megakaryocytes to induce the proliferation and differentiation of megakaryocytic progenitor cells from hematopoietic stem cells and megakaryocyte maturation.
12.3 Pharmacokinetics
Lusutrombopag demonstrated dose-proportional pharmacokinetics after single doses ranging from 1 mg (0.33 times the lowest approved dosage) to 50 mg (16.7 times the highest recommended dosage). Healthy subjects administered 3 mg of lusutrombopag had a geometric mean (%CV) maximal concentration (Cmax) of 111 (20.4) ng/mL and area under the time-concentration curve extrapolated to infinity (AUC0-inf) of 2931 (23.4) ng.hr/mL. The pharmacokinetics of lusutrombopag were similar in both healthy subjects and the chronic liver disease population.
The accumulation ratios of Cmax and AUC were approximately 2 with once-daily multiple-dose administration, and steady-state plasma lusutrombopag concentrations were achieved after Day 5.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In 2-year studies, lusutrombopag was not carcinogenic to rats at oral doses up to 20 mg/kg/day in males and 2 mg/kg/day in females (a dose 49 times and 30 times, respectively, the human exposure (AUC) at the recommended clinical dose of 3 mg/day for 7 days) and to mice at oral doses up to 20 mg/kg/day in males and females (a dose approximately 45 times the human exposure (AUC) at the recommended clinical dose of 3 mg/day for 7 days).
Lusutrombopag was not genotoxic based on an in vitro bacterial reverse mutation (Ames) assay, a chromosomal aberration assay with cultured Chinese hamster lung cells, and an in vivo micronucleus assay with mouse bone marrow cells.
In a fertility and early embryonic development study, lusutrombopag did not affect fertility in male and female rats at oral doses up to 100 mg/kg/day (a dose in males and females approximately 176 and 252 times, respectively, the human exposure (AUC) at the recommended clinical dose of 3 mg/day for 7 days).
14. Clinical Studies
The efficacy of MULPLETA for the treatment of thrombocytopenia in patients with chronic liver disease who are scheduled to undergo a procedure was evaluated in 2 randomized, double-blind, placebo-controlled trials (L-PLUS 1 (N=97) and L-PLUS 2 (N=215; NCT02389621)). Patients with chronic liver disease who were undergoing an invasive procedure and had a platelet count less than 50 × 109/L were eligible to participate. Patients undergoing laparotomy, thoracotomy, open-heart surgery, craniotomy, or organ resection were excluded. Patients with a history of splenectomy, partial splenic embolization, or thrombosis and those with Child-Pugh class C liver disease, absence of hepatopetal blood flow, or a prothrombotic condition other than chronic liver disease were not allowed to participate.
The patient populations were similar between the MULPLETA and placebo arms and consisted of 60% male and 40% female; median age was 60 years (range 19-88). The racial and ethnic distribution was White (55%), Asian (41%), and Other (4%).
Patients were randomized 1:1 to receive 3 mg of MULPLETA or placebo once daily for up to 7 days. Randomization was stratified by liver ablation/coagulation or other procedures and the platelet count at screening/baseline. In L-PLUS 1, 57% of patients underwent procedures other than liver ablation/coagulation and 43% underwent liver ablation/coagulation (RFA/MCT). In L-PLUS 2, 98% of patients underwent procedures other than liver ablation/coagulation and 2% underwent liver ablation/coagulation (RFA/MCT). Procedures other than liver ablation/coagulation (RFA/MCT) included liver-related procedures (transcatheter arterial chemoembolization, liver biopsy, and others), upper and lower gastrointestinal endoscopy-related procedures (endoscopic variceal ligation, endoscopic injection sclerotherapy, polypectomy, and biopsy), and other procedures (dental extraction, diagnostic paracentesis or laparocentesis, septoplasty, embolization of splenic artery aneurysm, bone marrow biopsy, removal of cervical polyp, and inguinal hernia repair (non-laparotomy based)).
In L-PLUS 1, the major efficacy outcome was the proportion of patients who require no platelet transfusion prior to the primary invasive procedure. In L-PLUS 2, the major efficacy outcome was the proportion of patients who require no platelet transfusion prior to the primary invasive procedure and no rescue therapy for bleeding (i.e., platelet preparations, other blood preparations, including red blood cells and plasma, volume expanders) from randomization through 7 days after the primary invasive procedure. In both trials, additional efficacy outcomes included the proportion of patients who require no platelet transfusion during the study, proportion of responders, duration of the increase in platelet count defined as the number of days during which the platelet count was maintained as ≥50 × 109/L, and the time course of platelet counts.
In both the L-PLUS 1 and L-PLUS 2 trials, responders were defined as patients who had a platelet count of ≥50 × 109/L with an increase of ≥20 × 109/L from baseline.
| Endpoint | Proportion (n/N) Exact 95% Confidence Interval | Treatment Difference (95% Confidence Interval) p value |
|
|---|---|---|---|
| MULPLETA (N=49) | Placebo (N=48) |
||
|
|||
| Not requiring platelet transfusion prior to invasive procedure* | 78% (38/49) (63, 88) | 13% (6/48) (4.7, 25) | 64 (49, 79) <0.0001† |
| Responder‡ during study | 76% (37/49) (61, 87) | 6% (3/48) (1.3, 17) | 68 (54, 82) <0.0001† |
| Endpoint | Proportion (n/N) Exact 95% Confidence Interval | Treatment Difference (95% Confidence Interval) p value |
|
|---|---|---|---|
| MULPLETA (N=108) | Placebo (N=107) |
||
|
|||
| Not requiring platelet transfusion prior to invasive procedure* or rescue therapy for bleeding from randomization through 7 days after invasive procedure | 65% (70/108) (55, 74) | 29% (31/107) (21, 39) | 37 (25, 49) <0.0001† |
| Responder‡ during study | 65% (70/108) (55, 74) | 13% (14/107) (7.3, 21) | 52 (41, 62) <0.0001† |
The median (Q1, Q3) duration of platelet count increase to at least 50 × 109/L was 22 (17, 27) days in MULPLETA-treated patients without platelet transfusion and 1.8 (0.0, 8.3) days in placebo-treated patients with platelet transfusion in L-PLUS 1 and 19 (13, 28) days in MULPLETA-treated patients without platelet transfusion and 0.0 (0.0, 5.0) days in placebo-treated patients with platelet transfusion in L-PLUS 2.
16. How is Mulpleta supplied
MULPLETA is supplied as 3 mg lusutrombopag tablets in a child-resistant blister pack containing 7 tablets - NDC 59630-551-07.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Prior to treatment, patients should fully understand and be informed of the following risks and considerations for MULPLETA.
| PATIENT INFORMATION MULPLETA® (mul ple' tah) (lusutrombopag) Tablets |
|
|---|---|
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Issued: 4/2020 |
| What is MULPLETA? | |
| MULPLETA is a prescription medicine used to treat low platelet count (thrombocytopenia) in adults with chronic liver disease who are scheduled to have a procedure. | |
| MULPLETA is not used to make platelet count normal in people with chronic liver disease. | |
| It is not known if MULPLETA is safe and effective in children. | |
Before taking MULPLETA, tell your healthcare provider about all of your medical conditions, including if you:
|
|
| Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. | |
How should I take MULPLETA?
|
|
| What are the possible side effects of MULPLETA? | |
| MULPLETA may cause serious side effects, including: | |
| Blood clots, including blood clots in the liver, may happen in people with chronic liver disease and who take MULPLETA. You may have an increased risk of blood clots if you have certain blood clotting conditions. | |
| The most common side effect of MULPLETA is headache. | |
| These are not all of the possible side effects of MULPLETA. | |
| Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. | |
How should I store MULPLETA?
|
|
| Keep MULPLETA and all medicines out of the reach of children. | |
| General information about the safe and effective use of MULPLETA. | |
| Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use MULPLETA for a condition for which it was not prescribed. Do not give MULPLETA to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about MULPLETA that is written for healthcare professionals. | |
| What are the ingredients of MULPLETA? | |
| Active ingredient: lusutrombopag. | |
| Inactive ingredients: D-mannitol, microcrystalline cellulose, magnesium oxide, sodium lauryl sulfate, hydroxypropyl cellulose, carboxymethylcellulose calcium, magnesium stearate, hypromellose, triethyl citrate, titanium dioxide, red ferric oxide, and talc. | |
| Manufactured for: Shionogi Inc., Florham Park, NJ 07932 | |
| © Shionogi Inc. 2020 | |
| For more information, go to www.mulpleta.com or call 1-800-849-9707. | |
| MULPLETA
lusutrombopag tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - SHIONOGI INC. (098241610) |






