Drug Detail:Multihance (Gadobenate dimeglumine [ gad-oh-ben-ate-dye-meg-loo-meen ])
Drug Class: Magnetic resonance imaging contrast media
Highlights of Prescribing Information
MultiHance (gadobenate dimeglumine) Injection
Initial U.S. Approval: 2004
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
See full prescribing information for complete boxed warning
Gadolinium-based contrast agents (GBCAs) increase the risk for NSF among patients with impaired elimination of the drugs. Avoid use of GBCAs in these patients unless the diagnostic information is essential and not available with non-contrast MRI or other modalities.
-
The risk for NSF appears highest among patients with:
- chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or
- acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age >60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing. (5.1)
Recent Major Changes
Indications and Usage for MultiHance
- magnetic resonance imaging (MRI) of the central nervous system (CNS) in adults and pediatric patients (including term neonates), to visualize lesions with abnormal blood-brain barrier or abnormal vascularity of the brain, spine, and associated tissues. (1.1)
- magnetic resonance angiography (MRA) to evaluate adults with known or suspected renal or aorto-ilio-femoral occlusive vascular disease. (1.2)
MultiHance Dosage and Administration
- The recommended dose of MultiHance is 0.2 mL/kg (0.1 mmol/kg) administered as a rapid bolus intravenous injection.
- For MRI of the CNS in pediatric patients below 2 years of age the recommended dosage range is 0.1 to 0.2 mL/kg.
- To ensure complete injection of the contrast medium, follow the injection with a saline flush of at least 5 mL in MRI of the CNS and at least 20 mL in MRA. (2)
Dosage Forms and Strengths
Each mL of MultiHance Injection contains 529 mg gadobenate dimeglumine and is available in single use vials. (3)
Contraindications
MultiHance is contraindicated in patients with known allergic or hypersensitivity reactions to gadolinium-based contrast agents. (4)
Warnings and Precautions
- Nephrogenic Systemic Fibrosis has occurred in patients with impaired elimination of GBCAs. Higher than recommended dosing or repeated dosing appears to increase the risk. (5.1)
- Hypersensitivity: anaphylactic/anaphylactoid reactions with cardiovascular, respiratory and cutaneous manifestations, ranging from mild to severe reactions including shock can occur. Monitor patients closely for need of emergency cardiorespiratory support. (5.2)
- Gadolinium is retained for months or years in brain, bone, and other organs. (5.3)
Adverse Reactions/Side Effects
The most commonly reported adverse reactions are nausea (1.3%) and headache (1.2%). (6)
To report SUSPECTED ADVERSE REACTIONS, contact Bracco Diagnostics Inc. at 1-800-257-5181 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
Use In Specific Populations
Pregnancy: Use only if imaging is essential during pregnancy and cannot be delayed. (8.1)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 8/2018
Full Prescribing Information
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
-
The risk for NSF appears highest among patients with:
- chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or
- acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended MultiHance dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration. [see Warnings and Precautions (5.1)]
Indications and Usage for MultiHance
MultiHance Dosage and Administration
2.1 Dosing and Imaging Instructions
2.2 Dosing Table
| *For pediatric patients less than 2 years of age, one-half of the per kg dose may be used. | ||
| TABLE 1: WEIGHT-BASED DOSING VOLUMES FOR: CNS IMAGING (ADULTS AND PEDIATRICS ≥2 YEARS OF AGE*) AND MRA IMAGING (ADULTS ONLY) |
||
| 0.1mM/kg dose | ||
| Kilograms (Kg) | Pounds (lb) | Volume, Milliliters (mL) |
| 2.5 | 5.5 | 0.5 |
| 5 | 11 | 1.0 |
| 10 | 22 | 2.0 |
| 15 | 33 | 3.0 |
| 20 | 44 | 4.0 |
| 25 | 55 | 5.0 |
| 30 | 66 | 6.0 |
| 35 | 77 | 7.0 |
| 40 | 88 | 8.0 |
| 45 | 99 | 9.0 |
| 50 | 110 | 10.0 |
| 55 | 121 | 11.0 |
| 60 | 132 | 12.0 |
| 65 | 143 | 13.0 |
| 70 | 154 | 14.0 |
| 75 | 165 | 15.0 |
| 80 | 176 | 16.0 |
| 85 | 187 | 17.0 |
| 90 | 198 | 18.0 |
| 95 | 209 | 19.0 |
| 100 | 220 | 20.0 |
| 105 | 231 | 21.0 |
| 110 | 242 | 22.0 |
| 115 | 253 | 23.0 |
| 120 | 264 | 24.0 |
| 125 | 275 | 25.0 |
| 130 | 286 | 26.0 |
| 135 | 297 | 27.0 |
| 140 | 308 | 28.0 |
| 145 | 319 | 29.0 |
| 150 | 330 | 30.0 |
Warnings and Precautions
Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the label:
- Nephrogenic systemic fibrosis [see Warnings and Precautions (5.1)]
- Hypersensitivity reactions [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
| TABLE 2: ADVERSE REACTIONS REPORTED IN ≥ 0.5% OF ADULT SUBJECTS WHO RECEIVED MULTIHANCE IN CLINICAL TRIALS | |
| Number of subjects dosed | 4967 |
| Number of subjects with any adverse reaction | 517 (10.4%) |
| Gastrointestinal
Disorders Nausea | 67 (1.3%) |
| General Disorders
and Administration Site Disorders Injection Site Reaction Feeling Hot | 54 (1.1%) 49 (1.0%) |
| Nervous System
Disorders Headache Dysgeusia Paresthesia Dizziness | 60 (1.2%) 33 (0.7%) 24 (0.5%) 24 (0.5%) |
MultiHance Description
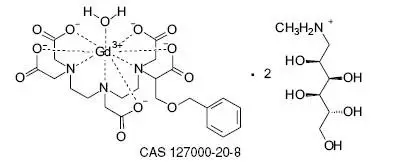
MultiHance has a pH of 6.5-7.5. Pertinent physicochemical parameters are provided below:
| Osmolality | 1.970 osmol/kg @ 37°C |
| Viscosity | 5.3 mPas @ 37°C |
| Density | 1.220 g/mL @ 20°C |
MultiHance - Clinical Pharmacology
12.2 Pharmacodynamics
| r1 and
r2 relaxivities indicate the efficiency in
shortening T1 and T2 relaxation times, respectively. 1 In heparinized human plasma, at 39°C. 2 In citrated human plasma, at 37°C. -- Not available |
||
| Human plasma | ||
| r1 | r2 | |
| Gadobenate | 9.71 | 12.51 |
| Gadopentetate | 4.91 | 6.31 |
| Gadodiamide | 5.42 | -- |
| Gadoteridol | 5.42 | -- |
Clinical Studies
14.1 MRI of the CNS
| (a)
Difference of means = (paired mean) - (pre mean) (b) Worse = paired score is less than the pre score Same = paired score is the same as the pre score Better = paired score is greater than the pre score * Statistically significant for the mean (paired t test) |
||||||
| Table 4: LESION LEVEL RESULTS OF MRI CENTRAL NERVOUS SYSTEM ADULT STUDIES WITH 0.1 mmol/kg MULTIHANCE | ||||||
| Study A | Study B | |||||
| Reader 1 | Reader 2 | Reader 3 | Reader 1 | Reader 2 | Reader 3 | |
| Endpoints | N = 395 | N = 384 | N = 299 | N = 245 | N = 275 | N = 254 |
| Border Delineation: | ||||||
| Difference of Means (a) | 0.8* | 0.6* | 0.8* | 1.8* | 1.5* | 1.9* |
| Worse (b) Same Better | 44 (11%) 146 (37%) 205 (52%) | 61 (16%) 168 (44%) 155 (40%) | 57 (19%) 89 (30%) 153 (51%) | 13 (5%) 11 (5%) 221 (90%) | 24 (9%) 19 (7%) 232 (84%) | 15 (6%) 18 (7%) 221 (87%) |
| Internal Morphology: | ||||||
| Difference of Means | 0.8* | 0.6* | 0.7* | 1.7* | 1.4* | 2.1* |
| Worse Same Better | 37 (10%) 147 (37%) 211 (53%) | 63 (17%) 151 (39%) 170 (44%) | 62 (21%) 84 (28%) 153 (51%) | 13 (5%) 16 (7%) 216 (88%) | 26 (10%) 22 (8%) 227 (82%) | 14 (5%) 22 (9%) 218 (86%) |
| Contrast Enhancement: | ||||||
| Difference of Means | 0.7* | 0.5* | 0.8* | 1.9* | 1.3* | 1.9* |
| Worse Same Better | 75 (19%) 148 (37%) 172 (44%) | 74 (19%) 152 (40%) 158 (41%) | 50 (17%) 109 (36%) 140 (47%) | 13 (5%) 11 (5%) 221 (90%) | 32 (12%) 21 (7%) 222 (81%) | 17 (7%) 14 (5%) 223 (88%) |
14.2 MRA of Renal and Aorto-ilio-femoral Vessels
| TABLE 5. PERFORMANCE CHARACTERISTICS OF MULTIHANCE-MRA AND NON-CONTRAST MRA | ||||||
| * (Based on General Delta Method) | ||||||
| READER | AORTO-ILIO-FEMORAL ARTERIES | |||||
| SENSITIVITY | SPECIFICITY | |||||
| MultiHance MRA [A] | Non-contrast MRA [B] | [A] – [B] (95% CI)* | MultiHance MRA [A] | Non-contrast MRA [B] | [A] – [B] (95% CI)* |
|
| 1 | 77.8% | 73.7% | 4.5 (1.5, 7.6) | 88.1% | 78.5% | 10.0 (7.3, 12.6) |
| 2 | 65.2% | 52.5% | 12.6 (8.5, 16.6) | 94.2% | 89.4% | 4.9 (2.7, 7.1) |
| 3 | 69.0% | 59.1% | 10.0 (6.1, 14.0) | 90.0% | 75.3% | 14.9 (12.1, 17.8) |
| READER | RENAL ARTERIES | |||||
| SENSITIVITY | SPECIFICITY | |||||
| MultiHance MRA [A] | Non-contrast MRA [B] | [A] – [B] (95% CI)* | MultiHance MRA [A] | Non-contrast MRA [B] | [A] – [B] (95% CI)* |
|
| 1 | 67.8% | 47.0% | 20.8 (12.8, 28.9) | 94.0% | 86.1% | 8.3 (4.2, 12.4) |
| 2 | 62.4% | 46.7% | 16.2 (6.8, 25.6) | 94.0% | 83.5% | 10.3 (5.5, 15.0) |
| 3 | 65.5% | 39.6% | 25.3 (15.9, 34.6) | 94.7% | 87.3% | 8.0 (3.6, 12.5) |
Patient Counseling Information
- Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Nephrogenic Systemic
Fibrosis
Instruct patients to inform their physician
if they:
- have a history of kidney and/or liver disease, or
- have recently received a GBCA.
- Describe the clinical manifestations of NSF
- Describe procedures to screen for the detection of renal impairment.
Common
Adverse Reactions
Inform patients that they may
experience:
- reactions along the venous injection site, such as mild and transient burning or pain or feeling of warmth or coldness at the injection site
- side effects of feeling hot, nausea, and headache.
Rx only
US Patent No. 4,916,246
Manufactured for
Bracco Diagnostics
Inc.
Monroe Township, NJ 08831
Medication Guide
| This Medication Guide has been approved by the U.S. Food and Drug Administration | Issued 04/2018 COEB403 |
| MEDICATION GUIDE
MULTIHANCE®(məl-tē-han(t)s) (gadobenate dimeglumine) Injection for intravenous use |
|
What is MULTIHANCE?
|
|
What is the
most important information I should know about MULTIHANCE?
|
|
| Do not receive MULTIHANCE if you have had a severe allergic reaction to GBCAs including gadobenate dimeglumine, or any of the ingredients in MULTIHANCE. | |
Before receiving
MULTIHANCE, tell your healthcare provider about all your medical conditions,
including if you:
|
|
What are the
possible side effects of MULTIHANCE?
These are not all the possible side effects of MULTIHANCE. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|
| General information
about the safe and effective use of MULTIHANCE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your healthcare provider for information about MULTIHANCE that is written for health professionals. |
|
| What are the
ingredients in MULTIHANCE? Active ingredient: gadobenate dimeglumine Inactive ingredients: water Manufactured by: BIPSO GmbH-78224 Singen (Germany) Manufactured for: Bracco Diagnostics Inc., Monroe Township, NJ 08831 US Patent No. 4,916,246 For more information, go to www.imaging.bracco.com or call 1-800-257-5181. |
|
| MULTIHANCE
gadobenate dimeglumine injection, solution |
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BRACCO DIAGNOSTICS INC (849234661) |
| Registrant - BRACCO DIAGNOSTICS INC (849234661) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BRACCO IMAGING SPA | 434384007 | API MANUFACTURE(0270-5164) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BIPSO GmbH | 342104149 | MANUFACTURE(0270-5164) , ANALYSIS(0270-5164) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Labor LS SE & Co. KG | 314929072 | ANALYSIS(0270-5164) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| PATHEON ITALIA SPA | 434078638 | MANUFACTURE(0270-5164) , ANALYSIS(0270-5164) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BioChem Labor für biologishe und chemische Analytik GmbH | 318354230 | ANALYSIS(0270-5164) | |