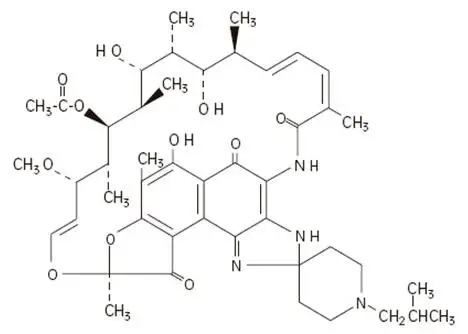
Drug Detail:Mycobutin (Rifabutin [ rif-a-bue-tin ])
Drug Class: Rifamycin derivatives
Mycobutin - Clinical Pharmacology
Microbiology
In Vitro Studies
Rifabutin has demonstrated in vitro activity against M. avium complex (MAC) organisms isolated from both HIV-positive and HIV-negative people. While-gene probe techniques may be used to identify these two organisms, many reported studies did not distinguish between these two species. The vast majority of isolates from MAC-infected, HIV-positive people are M. avium, whereas in HIV-negative people, about 40% of the MAC isolates are M. intracellulare.
Various in vitro methodologies employing broth or solid media, with and without polysorbate 80 (Tween 80), have been used to determine rifabutin MIC values for mycobacterial species. In general, MIC values determined in broth are several fold lower than that observed with methods employing solid media. Utilization of Tween 80 in these assays has been shown to further lower MIC values.
However, MIC values were substantially higher for egg-based compared to agar-based solid media.
Rifabutin activity against 211 MAC isolates from HIV-positive people was evaluated in vitro utilizing a radiometric broth and an agar dilution method. Results showed that 78% and 82% of these isolates had MIC99 values of ≤0.25 µg/mL and ≤1.0 µg/mL, respectively, when evaluated by these two methods. Rifabutin was also shown to be active against phagocytized, M. avium complex in a mouse macrophage cell culture model.
Rifabutin has in vitro activity against many strains of Mycobacterium tuberculosis. In one study, utilizing the radiometric broth method, each of 17 and 20 rifampin-naive clinical isolates tested from the United States and Taiwan, respectively, were shown to be susceptible to rifabutin concentrations of ≤0.125 µg/mL.
Cross-resistance between rifampin and rifabutin is commonly observed with M. tuberculosis and M. avium complex isolates. Isolates of M. tuberculosis resistant to rifampin are likely to be resistant to rifabutin. Rifampicin and rifabutin MIC99 values against 523 isolates of M. avium complex were determined utilizing the agar dilution method (Heifets, Leonid B. and Iseman, Michael D. Determination of in vitro susceptibility of Mycobacteria to Ansamycin. Am. Rev. Respir. Dis. 1985; 132(3):710–711).
| % of Strains Susceptible/Resistant to Different Concentrations of Rifabutin (μg/mL) | |||||
|---|---|---|---|---|---|
| Susceptibility to Rifampin (µg/mL) | Number of Strains | Susceptible to 0.5 | Resistant to 0.5 only | Resistant to 1.0 | Resistant to 2.0 |
| Susceptible to 1.0 | 30 | 100.0 | 0.0 | 0.0 | 0.0 |
| Resistant to 1.0 only | 163 | 88.3 | 11.7 | 0.0 | 0.0 |
| Resistant to 5.0 | 105 | 38.0 | 57.1 | 2.9 | 2.0 |
| Resistant to 10.0 | 225 | 20.0 | 50.2 | 19.6 | 10.2 |
| TOTAL | 523 | 49.5 | 36.7 | 9.0 | 4.8 |
Rifabutin in vitro MIC99 values of ≤0.5 µg/mL, determined by the agar dilution method, for M. kansasii, M. gordonae and M. marinum have been reported; however, the clinical significance of these results is unknown.
Precautions
Drug Interactions
Effect of Other Drugs on Rifabutin Pharmacokinetics
Some drugs that inhibit CYP3A may significantly increase the plasma concentration of rifabutin. Therefore, carefully monitor for rifabutin associated adverse events in those patients also receiving CYP3A inhibitors, which include fluconazole and clarithromycin. In some cases, the dosage of MYCOBUTIN may need to be reduced when it is coadministered with CYP3A inhibitors.
Table 2 summarizes the results and magnitude of the pertinent drug interactions assessed with rifabutin. The clinical relevance of these interactions and subsequent dose modifications should be judged in light of the population studied, severity of the disease, patient's drug profile, and the likely impact on the risk/benefit ratio.
| Coadministered drug | Dosing regimen of coadministered drug | Dosing regimen of rifabutin | Study population (n) | Effect on rifabutin | Effect on coadministered drug | Recommendation |
|---|---|---|---|---|---|---|
| ↑ indicates increase; ↓ indicates decrease; ↔ indicates no significant change ND - No Data AUC - Area under the Concentration vs. Time Curve; Cmax - Maximum serum concentration; Cmin – Minimum serum concentration |
||||||
|
||||||
| ANTIRETROVIRALS | ||||||
| Amprenavir | 1200 mg twice a day for 10 days | 300 mg once a day for 10 days | Healthy male subjects (6) | ↑ AUC by 193%, ↑ Cmax by 119% | ↔ | Reduce rifabutin dose by at least 50%. Monitor closely for adverse reactions. |
| Bictegravir | 75 mg once a day | 300 mg once a day (fasted) | Healthy subjects | ND | ↓ AUC 38% ↓ Cmin 56% ↓ Cmax 20% | Co-administration of rifabutin with Biktarvy (bictegravir/emtricitabine/tenofovir alafenamide) is not recommended due to an expected decrease in tenofovir alafenamide in addition to the reported reduction in bictegravir. Refer to Biktarvy prescribing information for additional information |
| Delavirdine | 400 mg three times a day | 300 mg once a day | HIV-infected patients (7) | ↑ AUC by 230%, ↑ Cmax by 128% | ↓ AUC by 80%, ↓ Cmax by 75%, ↓ Cmin by 17% | CONTRAINDICATED |
| Didanosine | 167 or 250 mg twice a day for 12 days | 300 or 600 mg once a day for 12 days | HIV-infected patients (11) | ↔ | ↔ | |
| Doravirine | 100 mg single dose | 300 mg once a day for 16 days | Healthy subjects (12) | ND | ↓ 50% in AUC, ↓ 68% in C24 ↔ in Cmax | If concomitant use is necessary, increase the doravirine dosage as instructed in doravirine-containing product prescribing information. |
| Fosamprenavir/ ritonavir | 700 mg twice a day plus ritonavir 100 mg twice a day for 2 weeks | 150 mg every other day for 2 weeks | Healthy subjects (15) | ↔ AUC*
↓ Cmax by 15% | ↑ AUC by 35%†, ↑ Cmax by 36%, ↑ Cmin by 36% | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with fosamprenavir/ritonavir combination. |
| Indinavir | 800 mg three times a day for 10 days | 300 mg once a day for 10 days | Healthy subjects (10) | ↑ AUC by 173%, ↑ Cmax by 134% | ↓ AUC by 34%, ↓ Cmax by 25%, ↓ Cmin by 39% | Reduce rifabutin dose by 50%, and increase indinavir dose from 800 mg to 1000 mg three times a day. |
| Lopinavir/ ritonavir | 400/100 mg twice a day for 20 days | 150 mg once a day for 10 days | Healthy subjects (14) | ↑ AUC by 203% ‡
↓ Cmax by 112% | ↔ | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with lopinavir/ritonavir combination. Monitor closely for adverse reactions. Reduce rifabutin dosage further, as needed. |
| Saquinavir/ ritonavir | 1000/100 mg twice a day for 14 or 22 days | 150 mg every 3 days for 21–22 days | Healthy subjects | ↑ AUC by 53% §
↑ Cmax by 88% (n=11) | ↓ AUC by 13%, ↓ Cmax by 15%, (n=19) | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with saquinavir/ritonavir combination. Monitor closely for adverse reactions. |
| Rilpivirine | 25 mg once a day | 300 mg once a day | Healthy subjects (18) | ND | ↓ AUC by 42% ↓ Cmin by 48% ↓ Cmax by 31% | Co-administration of rifabutin with Odefsey (rilpivirine/tenofovir alafenamide/emtricitabine) is not recommended, due to an expected decrease in tenofovir alafenamide in addition to the reported reduction in rilpivirine. Refer to Odefsey prescribing information for additional information. |
| Ritonavir | 500 mg twice a day for 10 days | 150 mg once a day for 16 days | Healthy subjects (5) | ↑ AUC by 300%, ↑ Cmax by 150% | ND | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with lopinavir/ritonavir combination. Monitor closely for adverse reactions. Reduce rifabutin dosage further, as needed. |
| Tipranavir/ ritonavir | 500/200 twice a day for 15 doses | 150 mg single dose | Healthy subjects (20) | ↑ AUC by 190%, ↑ Cmax by 70% | ↔ | Reduce rifabutin dose by at least 75% (to a maximum 150 mg every other day or three times per week) when given with tipranavir/ritonavir combination. Monitor closely for adverse reactions. Reduce rifabutin dosage further, as needed. |
| Nelfinavir | 1250 mg twice a day for 7–8 days | 150 mg once a day for 8 days | HIV-infected patients (11) | ↑ AUC by 83%, ¶
↑ Cmax by 19% | ↔ | Reduce rifabutin dose by 50% (to 150 mg once a day) and increase the nelfinavir dose to 1250 mg twice a day. |
| Zidovudine | 100 or 200 mg every four hours | 300 or 450 mg once a day | HIV-infected patients (16) | ↔ | ↓ AUC by 32%, ↓ Cmax by 48%, | Because zidovudine levels remained within the therapeutic range during co-administration of rifabutin, dosage adjustments are not necessary. |
| ANTIFUNGALS | ||||||
| Fluconazole | 200 mg once a day for 2 weeks | 300 mg once a day for 2 weeks | HIV-infected patients (12) | ↑ AUC by 82%, ↑ Cmax by 88% | ↔ | Monitor for rifabutin associated adverse events. Reduce rifabutin dose or suspend MYCOBUTIN use if toxicity is suspected. |
| Posaconazole | 200 mg once a day for 10 days | 300 mg once a day for 17 days | Healthy subjects (8) | ↑ AUC by 72%, ↑ Cmax by 31% | ↓ AUC by 49%, ↓ Cmax by 43% | If co-administration of these two drugs cannot be avoided, patients should be monitored for adverse events associated with rifabutin administration, and lack of posaconazole efficacy. |
| Itraconazole | 200 mg once a day | 300 mg once a day | HIV-Infected patients (6) | ↑# | ↓ AUC by 70%, ↓ Cmax by 75%, | If co-administration of these two drugs cannot be avoided, patients should be monitored for adverse events associated with rifabutin administration, and lack of itraconazole efficacy. In a separate study, one case of uveitis was associated with increased serum rifabutin levels following co-administration of rifabutin (300 mg once a day) with itraconazole (600–900 mg once a day). |
| Voriconazole | 400 mg twice a day for 7 days (maintenance dose) | 300 mg once a day for 7 days | Healthy male subjects (12) | ↑ AUC by 331%, ↑ Cmax by 195% | ↑ AUC by ~100%, ↑ Cmax by ~100%Þ | CONTRAINDICATED |
| ANTI-PCP (Pneumocystis carinii pneumonia) | ||||||
| Dapsone | 50 mg once a day | 300 mg once a day | HIV-infected patients (16) | ND | ↓ AUC by 27 –40% | |
| Sulfamethoxazole- Trimethoprim | 800/160 mg | 300 mg once a day | HIV-infected patients (12) | ↔ | ↓ AUC by 15–20% | |
| ANTI-MAC (Mycobacterium avium intracellulare complex) | ||||||
| Azithromycin | 500 mg once a day for 1 day, then 250 mg once a day for 9 days | 300 mg once a day | Healthy subjects (6) | ↔ | ↔ | |
| Clarithromycin | 500 mg twice a day | 300 mg once a day | HIV-infected patients (12) | ↑ AUC by 75% | ↓ AUC by 50% | Monitor for rifabutin associated adverse events. Reduce dose or suspend use of MYCOBUTIN if toxicity is suspected. Alternative treatment for clarithromycin should be considered when treating patients receiving rifabutin |
| ANTI-TB (Tuberculosis) | ||||||
| Ethambutol | 1200 mg | 300 mg once a day for 7 days | Healthy subjects (10) | ND | ↔ | |
| Isoniazid | 300 mg | 300 mg once a day for 7 days | Healthy subjects (6) | ND | ↔ | |
| OTHER | ||||||
| Methadone | 20 – 100 mg once a day | 300 mg once a day for 13 days | HIV-infected patients (24) | ND | ↔ | |
| Ethinylestradiol (EE)/Norethindrone (NE) | 35 mg EE / 1 mg NE for 21 days | 300 mg once a day for 10 days | Healthy female subjects (22) | ND | EE: ↓ AUC by 35%, ↓ Cmax by 20% NE: ↓ AUC by 46% | Patients should be advised to use additional or alternative methods of contraception. |
| Theophylline | 5 mg/kg | 300 mg for 14 days | Healthy subjects (11) | ND | ↔ | |
Adverse Reactions/Side Effects
Adverse Reactions from Clinical Trials
MYCOBUTIN Capsules were generally well tolerated in the controlled clinical trials. Discontinuation of therapy due to an adverse event was required in 16% of patients receiving MYCOBUTIN, compared to 8% of patients receiving placebo in these trials. Primary reasons for discontinuation of MYCOBUTIN were rash (4% of treated patients), gastrointestinal intolerance (3%), and neutropenia (2%).
The following table enumerates adverse experiences that occurred at a frequency of 1% or greater, among the patients treated with MYCOBUTIN in studies 023 and 027.
| Adverse event | MYCOBUTIN (n = 566) % | Placebo (n = 580) % |
|---|---|---|
| Body as a whole | ||
| Abdominal pain | 4 | 3 |
| Asthenia | 1 | 1 |
| Chest pain | 1 | 1 |
| Fever | 2 | 1 |
| Headache | 3 | 5 |
| Pain | 1 | 2 |
| Blood and lymphatic system | ||
| Leucopenia | 10 | 7 |
| Anemia | 1 | 2 |
| Digestive System | ||
| Anorexia | 2 | 2 |
| Diarrhea | 3 | 3 |
| Dyspepsia | 3 | 1 |
| Eructation | 3 | 1 |
| Flatulence | 2 | 1 |
| Nausea | 6 | 5 |
| Nausea and vomiting | 3 | 2 |
| Vomiting | 1 | 1 |
| Musculoskeletal system | ||
| Myalgia | 2 | 1 |
| Nervous system | ||
| Insomnia | 1 | 1 |
| Skin and appendages | ||
| Rash | 11 | 8 |
| Special senses | ||
| Taste perversion | 3 | 1 |
| Urogenital system | ||
| Discolored urine | 30 | 6 |
CLINICAL ADVERSE EVENTS REPORTED IN <1% OF PATIENTS WHO RECEIVED MYCOBUTIN
Considering data from the 023 and 027 pivotal trials, and from other clinical studies, MYCOBUTIN appears to be a likely cause of the following adverse events which occurred in less than 1% of treated patients: flu-like syndrome, hepatitis, hemolysis, arthralgia, myositis, chest pressure or pain with dyspnea, skin discoloration, thrombocytopenia, pancytopenia and jaundice.
The following adverse events have occurred in more than one patient receiving MYCOBUTIN, but an etiologic role has not been established: seizure, paresthesia, aphasia, confusion, and non-specific T wave changes on electrocardiogram.
When MYCOBUTIN was administered at doses from 1050 mg/day to 2400 mg/day, generalized arthralgia and uveitis were reported. These adverse experiences abated when MYCOBUTIN was discontinued.
Mild to severe, reversible uveitis has been reported less frequently when MYCOBUTIN is used at 300 mg as monotherapy in MAC prophylaxis versus MYCOBUTIN in combination with clarithromycin for MAC treatment (see also WARNINGS).
Uveitis has been infrequently reported when MYCOBUTIN is used at 300 mg/day as monotherapy in MAC prophylaxis of HIV-infected persons, even with the concomitant use of fluconazole and/or macrolide antibacterials. However, if higher doses of MYCOBUTIN are administered in combination with these agents, the incidence of uveitis is higher.
Patients who developed uveitis had mild to severe symptoms that resolved after treatment with corticosteroids and/or mydriatic eye drops; in some severe cases, however, resolution of symptoms occurred after several weeks.
When uveitis occurs, temporary discontinuance of MYCOBUTIN and ophthalmologic evaluation are recommended. In most mild cases, MYCOBUTIN may be restarted; however, if signs or symptoms recur, use of MYCOBUTIN should be discontinued (Morbidity and Mortality Weekly Report, September 9, 1994).
Corneal deposits have been reported during routine ophthalmologic surveillance of some HIV-positive pediatric patients receiving MYCOBUTIN as part of a multiple drug regimen for MAC prophylaxis. The deposits are tiny, almost transparent, asymptomatic peripheral and central corneal deposits, and do not impair vision.
The following table enumerates the changes in laboratory values that were considered as laboratory abnormalities in Studies 023 and 027.
| Laboratory abnormalities | MYCOBUTIN (n = 566) % | PLACEBO (n = 580) % |
|---|---|---|
| Includes grades 3 or 4 toxicities as specified: | ||
|
||
| Chemistry | ||
| Increased alkaline phosphatase * | <1 | 3 |
| Increased SGOT † | 7 | 12 |
| Increased SGPT † | 9 | 11 |
| Hematology | ||
| Anemia ‡ | 6 | 7 |
| Eosinophilia | 1 | 1 |
| Leukopenia § | 17 | 16 |
| Neutropenia ¶ | 25 | 20 |
| Thrombocytopenia # | 5 | 4 |
The incidence of neutropenia in patients treated with MYCOBUTIN was significantly greater than in patients treated with placebo (p = 0.03). Although thrombocytopenia was not significantly more common among patients treated with MYCOBUTIN in these trials, MYCOBUTIN has been clearly linked to thrombocytopenia in rare cases. One patient in Study 023 developed thrombotic thrombocytopenic purpura, which was attributed to MYCOBUTIN.
Mycobutin Dosage and Administration
It is recommended that MYCOBUTIN Capsules be administered at a dose of 300 mg once daily. For those patients with propensity to nausea, vomiting, or other gastrointestinal upset, administration of MYCOBUTIN at doses of 150 mg twice daily taken with food may be useful.
For patients with severe renal impairment (creatinine clearance less than 30 mL/min), consider reducing the dose of MYCOBUTIN by 50%, if toxicity is suspected. No dosage adjustment is required for patients with mild to moderate renal impairment. Reduction of the dose of MYCOBUTIN may also be needed for patients receiving concomitant treatment with certain other drugs (see PRECAUTIONS-Drug Interactions).
Mild hepatic impairment does not require a dose modification. The pharmacokinetics of rifabutin in patients with moderate and severe hepatic impairment is not known.
| MYCOBUTIN
rifabutin capsule |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Pfizer Laboratories Div Pfizer Inc (134489525) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Italia S.r.l. | 458521908 | ANALYSIS(0013-5301) , MANUFACTURE(0013-5301) , PACK(0013-5301) , LABEL(0013-5301) | |