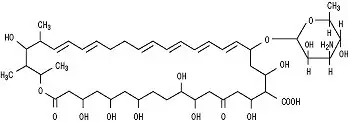
Drug Detail:Mycostatin topical (Nystatin topical [ nye-stat-in ])
Drug Class: Topical antifungals
Precautions
General
Nystatin, topical preparations should not be used for the treatment of systemic, oral, intravaginal or ophthalmic infections.
If irritation or sensitization develops, treatment should be discontinued and appropriate measures taken as indicated. It is recommended that KOH smears, cultures, or other diagnostic methods be used to confirm the diagnosis of cutaneous or mucocutaneous candidiasis and to rule out infection caused by other pathogens.
Mycostatin Dosage and Administration
Very moist lesions are best treated with the topical dusting powder.
MYCOSTATIN® Cream
Adults and Pediatric Patients (Neonates and Older):
Apply liberally to affected areas twice daily or as indicated until healing is complete.
MYCOSTATIN® Topical Powder
Adults and Pediatric Patients (Neonates and Older):
Apply to candidal lesions two or three times daily until healing is complete. For fungal infection of the feet caused by Candida species, the powder should be dusted on the feet, as well as, in all foot wear.
| MYCOSTATIN
nystatin cream |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| MYCOSTATIN
nystatin powder |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Bristol-Myers Squibb Company |