Drug Detail:Myfembree (Relugolix, estradiol and norethindrone acetate)
Drug Class: Sex hormone combinations
Highlights of Prescribing Information
MYFEMBREE® (relugolix, estradiol, and norethindrone acetate) tablets, for oral use
Initial U.S. Approval: 2021
WARNING: THROMBOEMBOLIC DISORDERS AND VASCULAR EVENTS
See full prescribing information for complete boxed warning
- Estrogen and progestin combinations, including MYFEMBREE, increase the risk of thrombotic or thromboembolic disorders, especially in women at increased risk for these events. (5.1)
- MYFEMBREE is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension. (4)
Recent Major Changes
| Indications and Usage, Moderate to Severe Pain Associated with Endometriosis, (1.2) | 08/2022 |
| Warnings and Precautions, Hypersensitivity Reactions, (5.14) | 01/2023 |
Indications and Usage for Myfembree
MYFEMBREE is a combination of relugolix, a gonadotropin-releasing hormone (GnRH) receptor antagonist, estradiol, an estrogen, and norethindrone acetate, a progestin, indicated in premenopausal women for the:
- management of heavy menstrual bleeding associated with uterine leiomyomas (fibroids). (1.1)
- management of moderate to severe pain associated with endometriosis. (1.2)
Limitations of Use
Use of MYFEMBREE should be limited to 24 months due to the risk of continued bone loss which may not be reversible. (1.3, 5.2, 6)
Myfembree Dosage and Administration
- Exclude pregnancy and discontinue hormonal contraceptives prior to MYFEMBREE initiation. (2.1)
- Take one tablet orally once daily. (2.2)
- Take the missed dose of MYFEMBREE as soon as possible the same day and then resume regular dosing the next day at the usual time. (2.3)
- If concomitant use of oral P-gp inhibitors is unavoidable, take MYFEMBREE at least 6 hours before taking the P-gp inhibitor (2.4)
Dosage Forms and Strengths
Tablets: fixed-dose combination containing relugolix 40 mg, estradiol 1 mg and norethindrone acetate 0.5 mg. (3)
Contraindications
- High risk of arterial, venous thrombotic, or thromboembolic disorder. (4)
- Pregnancy. (4)
- Known osteoporosis. (4)
- Current or history of breast cancer or other hormone-sensitive malignancies. (4)
- Known hepatic impairment or disease. (4)
- Undiagnosed abnormal uterine bleeding. (4)
- Known hypersensitivity to components of MYFEMBREE. (4)
Warnings and Precautions
- Thromboembolic Disorders and Vascular Events: Discontinue MYFEMBREE if an arterial or venous thrombotic, cardiovascular, or cerebrovascular event occurs. Discontinue MYFEMBREE if there is sudden unexplained partial or complete loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis immediately. (5.1)
- Bone Loss: Decreases in bone mineral density (BMD) may not be completely reversible. Baseline BMD assessment is recommended in all women. In women with heavy menstrual bleeding associated with uterine fibroids, periodic BMD assessments are recommended. In women with moderate to severe pain associated with endometriosis, annual BMD assessments are recommended. Assess risk-benefit for women with additional risk factors for bone loss. (5.2)
- Suicidal Ideation and Mood Disorders (Including Depression): Advise patients to seek medical attention for new onset or worsening depression, anxiety, or other mood changes. (5.4)
- Hepatic Impairment and Transaminase Elevations: Counsel patients on signs and symptoms of liver injury. (5.5)
- Elevated Blood Pressure: Do not use in women with uncontrolled hypertension. For women with well-controlled hypertension, continue to monitor blood pressure and stop MYFEMBREE if blood pressure rises significantly. (5.7)
- Change in Menstrual Bleeding Pattern and Reduced Ability to Recognize Pregnancy: Advise women to use non-hormonal contraception during treatment and for one week after discontinuing MYFEMBREE. MYFEMBREE may delay the ability to recognize pregnancy because it alters menstrual bleeding. Perform testing if pregnancy is suspected and discontinue MYFEMBREE if pregnancy is confirmed. (5.8)
- Risk of Early Pregnancy Loss: Can cause early pregnancy loss. Advise women to use effective non-hormonal contraception. (5.9)
- Uterine Fibroid Prolapse or Expulsion: Advise patients to seek medical attention for severe uterine bleeding. (5.10)
- Hypersensitivity Reactions: Immediately discontinue MYFEMBREE if a hypersensitivity reaction occurs. (5.14)
Adverse Reactions/Side Effects
In women with heavy menstrual bleeding associated with uterine fibroids, most common adverse reactions (incidence ≥ 3%) are vasomotor symptoms, uterine bleeding, alopecia, and decreased libido.
In women with moderate to severe pain associated with endometriosis, most common adverse reactions (incidence ≥ 3%) are headache, vasomotor symptoms, mood disorders, abnormal uterine bleeding, nausea, toothache, back pain, decreased sexual desire and arousal, arthralgia, fatigue, and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Myovant Sciences, Inc. at 1-833-MYOVANT (1-833-696-8268) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Avoid use of MYFEMBREE with oral P-gp inhibitors. (7.1)
- Avoid use with combined P-gp and strong CYP3A inducers, as the exposure of the components of MYFEMBREE may be decreased. (7.1)
Use In Specific Populations
- Lactation: Advise women not to breastfeed while taking MYFEMBREE. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 2/2023
Full Prescribing Information
WARNING: THROMBOEMBOLIC DISORDERS AND VASCULAR EVENTS
- Estrogen and progestin combination products, including MYFEMBREE, increase the risk of thrombotic or thromboembolic disorders including pulmonary embolism (PE), deep vein thrombosis (DVT), stroke and myocardial infarction (MI), especially in women at increased risk for these events [see Warnings and Precautions (5.1)].
- MYFEMBREE is contraindicated in women with current or a history of thrombotic or thromboembolic disorders and in women at increased risk for these events, including women over 35 years of age who smoke or women with uncontrolled hypertension [see Contraindications (4)].
1. Indications and Usage for Myfembree
1.1 Heavy Menstrual Bleeding Associated with Uterine Leiomyomas
MYFEMBREE is indicated for the management of heavy menstrual bleeding associated with uterine leiomyomas (fibroids) in premenopausal women.
2. Myfembree Dosage and Administration
2.1 Prior to Initiation of MYFEMBREE
- Exclude pregnancy [see Contraindications (4)].
- Discontinue hormonal contraceptives [see Warnings and Precautions (5.8)].
2.2 Recommended Dosage
- Take one tablet of MYFEMBREE orally once daily at approximately the same time, with or without food [see Clinical Pharmacology (12.3)].
- Start MYFEMBREE as early as possible after the onset of menses but no later than seven days after menses has started.
- The recommended total duration of treatment with MYFEMBREE is 24 months [see Indications and Usage (1.3) and Warnings and Precautions (5.2)].
4. Contraindications
MYFEMBREE is contraindicated in women:
- With a high risk of arterial, venous thrombotic, or thromboembolic disorders [see Boxed Warning and Warnings and Precautions (5.1)]. Examples include women over 35 years of age who smoke and women who are known to have:
- current or history of deep vein thrombosis or pulmonary embolism
- vascular disease (e.g., cerebrovascular disease, coronary artery disease, peripheral vascular disease)
- thrombogenic valvular or thrombogenic rhythm diseases of the heart (e.g., subacute bacterial endocarditis with valvular disease, or atrial fibrillation)
- inherited or acquired hypercoagulopathies
- uncontrolled hypertension
- headaches with focal neurological symptoms or migraine headaches with aura if over 35 years of age
- Who are pregnant. Exposure to MYFEMBREE early in pregnancy may increase the risk of early pregnancy loss [see Warnings and Precautions (5.9) and Use in Specific Populations (8.1)].
- With known osteoporosis, because of the risk of further bone loss [see Warnings and Precautions (5.2)].
- With current or history of breast cancer or other hormone-sensitive malignancies, and with increased risk for hormone-sensitive malignancies [see Warnings and Precautions (5.3)].
- With known hepatic impairment or disease [see Warnings and Precautions (5.5)].
- With undiagnosed abnormal uterine bleeding.
- With known anaphylactic reaction, angioedema, or hypersensitivity to MYFEMBREE or any of its components. Anaphylactoid reactions, urticaria, and angioedema have been reported [see Warnings and Precautions (5.14), Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Thromboembolic Disorders and Vascular Events
MYFEMBREE is contraindicated in women with current or history of thrombotic or thromboembolic disorders and in women at increased risk for these events [see Contraindications (4)].
Discontinue MYFEMBREE immediately if an arterial or venous thrombotic, cardiovascular, or cerebrovascular event occurs or is suspected. Discontinue MYFEMBREE at least 4 to 6 weeks before surgery of the type associated with an increased risk of thromboembolism, or during periods of prolonged immobilization, if feasible.
Discontinue MYFEMBREE immediately if there is sudden unexplained partial or complete loss of vision, proptosis, diplopia, papilledema, or retinal vascular lesions and evaluate for retinal vein thrombosis as these have been reported in patients receiving estrogens and progestins.
Estrogen and progestin combinations, including the estradiol/norethindrone acetate component of MYFEMBREE, increase the risk of thrombotic or thromboembolic disorders, including pulmonary embolism, deep vein thrombosis, stroke, and myocardial infarction, especially in women at high risk for these events. In general, the risk is greatest among women over 35 years of age who smoke and women with uncontrolled hypertension, dyslipidemia, vascular disease, or obesity.
Two thromboembolic events (DVT and PE) occurred in one woman treated for 38 days with MYFEMBREE for moderate to severe pain associated with endometriosis.
5.2 Bone Loss
MYFEMBREE is contraindicated in women with known osteoporosis [see Contraindications (4)]. Consider the benefits and risks of MYFEMBREE treatment in patients with a history of a low trauma fracture or risk factors for osteoporosis or bone loss, including taking medications that may decrease bone mineral density (BMD) (e.g., systemic or chronic inhaled corticosteroids, anticonvulsants, or chronic use of proton pump inhibitors).
Assessment of BMD by dual-energy X-ray absorptiometry (DXA) is recommended at baseline. In women with heavy menstrual bleeding associated with uterine fibroids, periodic DXA during treatment with MYFEMBREE is recommended. In women with moderate to severe pain associated with endometriosis, annual DXA is recommended while taking MYFEMBREE. Consider discontinuing MYFEMBREE if the risk associated with bone loss exceeds the potential benefit of treatment. Although the effect of supplementation with calcium and vitamin D was not studied, such supplementation for patients with inadequate dietary intake may be beneficial. MYFEMBREE may cause a decrease in BMD in some patients. BMD loss may be greater with increasing duration of use and may not be completely reversible after stopping treatment [see Adverse Reactions (6.1)]. The impact of BMD decreases on long-term bone health and future fracture risk in premenopausal women is unknown.
5.3 Hormone-Sensitive Malignancies
MYFEMBREE is contraindicated in women with current or a history of hormone-sensitive malignancies (e.g., breast cancer) and in women at increased risk for hormone-sensitive malignancies [see Contraindications (4)]. Discontinue MYFEMBREE if a hormone-sensitive malignancy is diagnosed.
Surveillance measures in accordance with standard of care, such as breast examinations and mammography, are recommended. The use of estrogen alone or estrogen plus progestin has been reported to result in an increase in abnormal mammograms requiring further evaluation.
5.4 Suicidal Ideation and Mood Disorders (Including Depression)
Evaluate patients with a history of suicidal ideation, depression, and mood disorders prior to initiating treatment. Monitor patients for mood changes and depressive symptoms including shortly after initiating treatment, to determine whether the risks of continuing therapy with MYFEMBREE outweigh the benefits. Patients with new or worsening depression, anxiety, or other mood changes should be referred to a mental health professional, as appropriate. Advise patients to seek immediate medical attention for suicidal ideation and behavior. Re-evaluate the benefits and risks of continuing MYFEMBREE if such events occur.
Gonadotropin-releasing hormone receptor antagonists, including MYFEMBREE, have been associated with mood disorders (including depression) and suicidal ideation.
In Studies L1 and L2 in women with heavy menstrual bleeding associated with uterine fibroids, a greater proportion of women treated with MYFEMBREE compared with placebo reported depression (including depression, mood swings, and depressed mood) (2.4% vs. 0.8%), irritability (2.4% vs. 0%), and anxiety (1.2% vs. 0.8%) [see Adverse Reactions (6.1)].
In Studies S1 and S2 in women with moderate to severe pain associated with endometriosis, a greater proportion of women treated with MYFEMBREE as compared to placebo reported mood disorders (including depression) (9.1% vs. 7.2%). In addition, cases of suicidal ideation were reported with MYFEMBREE use. All women who reported suicidal ideation had a history of depression and/or anxiety [see Adverse Reactions (6.1)].
5.6 Gallbladder Disease or History of Cholestatic Jaundice
Discontinue MYFEMBREE if signs or symptoms of gallbladder disease or jaundice occur. For women with a history of cholestatic jaundice associated with past estrogen use or with pregnancy, assess the risk-benefit of continuing therapy. Studies among estrogen users suggest a small increased relative risk of developing gallbladder disease.
5.7 Elevated Blood Pressure
MYFEMBREE is contraindicated in women with uncontrolled hypertension [see Contraindications (4)]. For women with well-controlled hypertension, continue to monitor blood pressure and stop MYFEMBREE if blood pressure rises significantly.
In the placebo-controlled clinical trials in women with heavy menstrual bleeding associated with uterine fibroids or with moderate to severe pain associated with endometriosis, more women in one study (Study L1; uterine fibroids) experienced the adverse reaction of new or worsening hypertension with MYFEMBREE compared to placebo (7.0% vs. 0.8%).
5.8 Change in Menstrual Bleeding Pattern and Reduced Ability to Recognize Pregnancy
Exclude pregnancy before initiating MYFEMBREE [see Dosage and Administration (2.1)]. Start MYFEMBREE as early as possible after the start of menses but no later than 7 days after menses has started. If MYFEMBREE is initiated later in the menstrual cycle, irregular and/or heavy bleeding may initially occur. Women who take MYFEMBREE may experience amenorrhea or a reduction in the amount, intensity, or duration of menstrual bleeding, which may delay the ability to recognize pregnancy. Perform pregnancy testing if pregnancy is suspected and discontinue MYFEMBREE if pregnancy is confirmed [see Use in Specific Populations (8.1, 8.3)].
Advise women of reproductive potential to use effective non-hormonal contraception during treatment with MYFEMBREE and for one week after the final dose. Avoid concomitant use of hormonal contraceptives with MYFEMBREE. The use of estrogen-containing hormonal contraceptives can increase estrogen levels which may increase the risk of estrogen-associated adverse events and decrease the efficacy of MYFEMBREE [see Use in Specific Populations (8.1, 8.3)].
5.9 Risk of Early Pregnancy Loss
MYFEMBREE is contraindicated for use in pregnancy [see Contraindications (4)]. Based on findings from animal studies and its mechanism of action, MYFEMBREE can cause early pregnancy loss. However, in both rabbits and rats, no fetal malformations were present at any dose level tested which were associated with relugolix exposures about half and approximately 300 times exposures in women at the recommended human dose [see Use in Specific Populations (8.1)].
5.10 Uterine Fibroid Prolapse or Expulsion
Advise women with known or suspected submucosal uterine fibroids about the possibility of uterine fibroid prolapse or expulsion and instruct them to contact their physician if severe bleeding and/or cramping occurs while being treated with MYFEMBREE. In Studies L1 and L2, uterine fibroid prolapse or uterine fibroid expulsion were reported in women treated with MYFEMBREE [see Adverse Reactions (6.1)].
5.11 Alopecia
Consider discontinuing MYFEMBREE if hair loss becomes a concern [see Adverse Reactions (6.1)].
In Phase 3 placebo-controlled clinical trials in women with heavy menstrual bleeding associated with uterine fibroids, 3.5% of MYFEMBREE-treated women experienced alopecia, hair loss, and hair thinning as compared to 0.8% of placebo-treated women. In 3 of the 11 affected women treated with MYFEMBREE across Studies L1 and L2, alopecia was reported as moderate. For one MYFEMBREE-treated woman in the extension trial, alopecia was a reason for discontinuing treatment. No specific pattern of hair loss was described. The majority of affected women completed the study with reported hair loss ongoing. Whether the hair loss is reversible is unknown [see Adverse Reactions (6.1)].
5.12 Effects on Carbohydrate and Lipid Metabolism
More frequent monitoring in MYFEMBREE-treated women with prediabetes and diabetes may be necessary. MYFEMBREE may decrease glucose tolerance and result in increased blood glucose concentrations.
Monitor lipid levels and consider discontinuing MYFEMBREE if hypercholesterolemia or hypertriglyceridemia worsens. In women with pre-existing hypertriglyceridemia, estrogen therapy may be associated with elevations in triglycerides levels leading to pancreatitis. Use of MYFEMBREE is associated with increases in total cholesterol and low-density lipoprotein cholesterol (LDL-C) [see Adverse Reactions (6.1)].
5.13 Effect on Other Laboratory Results
Patients with hypothyroidism and hypoadrenalism may require higher doses of thyroid hormone or cortisol replacement therapy.
The use of estrogen and progestin combinations may raise serum concentrations of binding proteins (e.g., thyroid-binding globulin, corticosteroid-binding globulin), which may reduce free thyroid or corticosteroid hormone levels.
The use of estrogen and progestin may also affect the levels of sex hormone-binding globulin, and coagulation factors [see Clinical Pharmacology (12.2)].
5.14 Hypersensitivity Reactions
Hypersensitivity reactions, including anaphylactoid reactions, urticaria and angioedema, have been reported with MYFEMBREE [see Adverse Reactions (6.2)]. MYFEMBREE is contraindicated in women with a history of hypersensitivity reactions to relugolix or any component of MYFEMBREE [see Contraindications (4)]. Immediately discontinue MYFEMBREE if a hypersensitivity reaction occurs.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are discussed elsewhere in the labeling:
- Thromboembolic Disorders and Vascular Events [see Warnings and Precautions (5.1)]
- Bone Loss [see Warnings and Precautions (5.2)]
- Suicidal Ideation and Mood Disorders (Including Depression) [see Warnings and Precautions (5.4)]
- Hepatic Impairment and Transaminase Elevations [see Warnings and Precautions (5.5)]
- Elevated Blood Pressure [see Warnings and Precautions (5.7)]
- Change in Menstrual Bleeding Pattern [see Warnings and Precautions (5.8)]
- Uterine Fibroid Prolapse or Expulsion [see Warnings and Precautions (5.10)]
- Alopecia [see Warnings and Precautions (5.11)]
- Effects on Carbohydrate and Lipid Metabolism [see Warnings and Precautions (5.12)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.14)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of MYFEMBREE, as well as post-approval use of relugolix monotherapy outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Immune system disorders: anaphylactoid reaction
Skin and subcutaneous tissue disorders: drug eruption, angioedema, urticaria
Neoplasms (benign, malignant, and unspecified): uterine leiomyoma degeneration
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
Based on animal data and the mechanism of action, MYFEMBREE can cause early pregnancy loss if MYFEMBREE is administered to pregnant women [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
Safety and effectiveness of MYFEMBREE in pediatric patients have not been established.
8.6 Hepatic Impairment
MYFEMBREE is contraindicated in women with hepatic impairment or disease [see Contraindications (4)]. The use of E2 (a component of MYFEMBREE) in patients with hepatic impairment is expected to increase the exposure to E2 and increase the risk of E2-associated adverse reactions [see Clinical Pharmacology (12.3)].
10. Overdosage
Overdosage of estrogen plus progestin may cause nausea, vomiting, breast tenderness, abdominal pain, drowsiness, fatigue, and withdrawal bleeding.
Supportive care is recommended if an overdose occurs. The amount of relugolix, estradiol, or norethindrone removed by hemodialysis is unknown.
11. Myfembree Description
MYFEMBREE tablets for oral administration contain a fixed-dose combination of relugolix 40 mg, estradiol 1 mg, and norethindrone acetate 0.5 mg as active ingredients.
Relugolix is a non-peptide small molecule, GnRH receptor antagonist. It is a white to off white to slightly yellow solid and is sparingly soluble in water. The chemical name is N-(4-{1-[(2,6-difluorophenyl)methyl]-5-[(dimethylamino)methyl]-3-(6-methoxypyridazin-3-yl)-2,4-dioxo-1,2,3,4-tetrahydrothieno[2,3-d]pyrimidin-6-yl}phenyl)-N-methoxyurea with the empirical formula of C29H27F2N7O5S and a molecular weight of 623.63. The structural formula is:
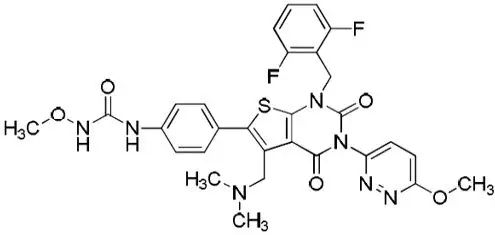
relugolix
Estradiol (E2), an estrogen, is present as the hemihydrate (C18H24O2∙½H2O) which is a white or almost white crystalline powder. Its chemical name is estra-1, 3, 5 (10)-triene-3, 17β-diol with the empirical formula of C18H24O2 and a molecular weight of 272.4. The structural formula is:
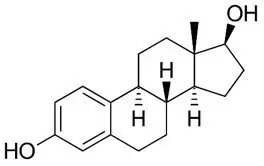
estradiol
Norethindrone acetate (NETA), a progestin, is a white or yellowish-white crystalline powder. Its chemical name is 17-Hydroxy-19-nor-17α-pregn-4-en-20-yn-3-one acetate with the empirical formula of C22H28O3 and a molecular weight of 340.5. The structural formula is:
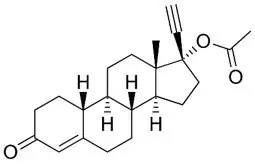
norethindrone acetate
Each MYFEMBREE (relugolix, estradiol, and norethindrone acetate) film-coated tablet contains the following inactive ingredients: hydroxypropyl cellulose, hypromellose, iron oxide yellow, lactose monohydrate, mannitol, magnesium stearate, sodium starch glycolate, titanium dioxide, and triacetin.
12. Myfembree - Clinical Pharmacology
12.1 Mechanism of Action
MYFEMBREE is a combination of relugolix, estradiol (E2), and norethindrone acetate (NETA).
Relugolix is a non-peptide GnRH receptor antagonist that competitively binds to pituitary GnRH receptors, thereby reducing the release of luteinizing hormone (LH) and follicle-stimulating hormone (FSH), leading to decreased serum concentrations of the ovarian sex hormones estradiol and progesterone and reduced bleeding associated with uterine fibroids and pain associated with endometriosis.
Estradiol acts by binding to nuclear receptors that are expressed in estrogen-responsive tissues. As a component of MYFEMBREE, the addition of exogenous estradiol may reduce the increase in bone resorption and resultant bone loss that can occur due to a decrease in circulating estrogen concentrations from relugolix alone.
Progestins such as norethindrone act by binding to nuclear receptors that are expressed in progesterone-responsive tissues. As a component of MYFEMBREE, norethindrone may protect the uterus from the potential adverse endometrial effects of unopposed estrogen.
12.2 Pharmacodynamics
Estradiol and norethindrone acetate (components of MYFEMBREE) may have the following effects:
- Increased thyroxin-binding globulin levels leading to [see Warnings and Precautions (5.13)]:
- Increased circulating total thyroid hormone concentrations as measured by protein-bound iodine (PBI), thyroxine (T4) levels (by column or by radioimmunoassay), or triiodothyronine (T3) concentrations by radioimmunoassay
- Decreased T3 resin uptake
- Unaltered free T4 and free T3 concentrations in women with normal thyroid function [see Warnings and Precautions (5.13)].
- Elevated corticosteroid-binding globulin (CBG) and sex hormone-binding globulin (SHBG) concentrations leading to increases in total circulating corticosteroid and sex hormone concentrations, respectively [see Warnings and Precautions (5.13)].
- Possible decreased free testosterone concentrations.
- Possible increased other plasma proteins concentrations (angiotensinogen/renin substrate, alpha-1 antitrypsin, ceruloplasmin).
- Increased plasma high-density lipoprotein (HDL) and HDL2 cholesterol subfraction concentration, reduced low-density lipoprotein concentration, increased triglyceride concentrations.
- Accelerated prothrombin time, partial thromboplastin time, and platelet aggregation time; increased platelet count; increased factors II, VII antigen, VIII coagulant activity, IX, X, XII, VII-X complex, and beta-thromboglobulin; decreased concentrations of anti-factor Xa and antithrombin III, decreased antithrombin III activity, increased concentrations of fibrinogen and fibrinogen activity; increased plasminogen antigen and activity.
12.3 Pharmacokinetics
The pharmacokinetic parameters of relugolix, unconjugated estradiol, and norethindrone after administration of a single dose of MYFEMBREE to healthy postmenopausal women under fasted conditions are summarized in Table 7.
| Relugolix | Unconjugated Estradiol | Norethindrone | |
|---|---|---|---|
| Abbreviations: AUC = area under the concentration-time curve; AUC0-inf = AUC from time 0 extrapolated to infinity; Cmax = maximum observed concentration; E2 = estradiol; NET = norethindrone; Tmax = time to maximum observed concentration. | |||
| Notes: AUC0-inf is presented in ng∙hr/mL for relugolix, NET and in pg∙hr/mL for unconjugated E2. Cmax is presented in ng/mL for relugolix, NET and in pg/mL for unconjugated E2. | |||
| AUC0-inf (ng∙hr/mL or pg∙hr/mL), mean (SD) | 198.1 (111.6) | 818.7 (334.4) | 17.5 (8.5) |
| Cmax (ng/mL or pg/mL), mean (SD) | 26.0 (18.2) | 28.0 (19.2) | 3.6 (1.4) |
| Tmax (hr), median (min, max) | 2.00 (0.25, 5.00) | 7.00 (0.25, 24.00) | 1.0 (0.50, 4.00) |
Relugolix exhibits greater than dose-proportional exposures at doses ranging from 1 mg to 80 mg (0.025 to 2 times the approved recommended dose) and approximately dose-proportional exposures at doses ranging from 80 mg to 360 mg (2 to 9 times the approved recommended dose). Relugolix concentrations reach steady-state within 12 days, and the degree of accumulation is approximately 2-fold, upon once daily administration.
Estradiol and norethindrone concentrations reach steady-state within 2 weeks, with an accumulation of 33% to 47% above concentrations seen after administration of a single dose, upon once daily administration.
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
Phospholipidosis (intracellular phospholipid accumulation) was observed in multiple organs and tissues (e.g., liver, pancreas, spleen, kidney, lymph nodes, lung, bone marrow, GI tract or testes) after repeated oral administration of relugolix in rats and monkeys. In a rat 26-week toxicity study, phospholipidosis was observed at doses of 100 mg/kg (approximately 30 times the exposure at the MRHD of 40 mg daily in women based on AUC) and above. In a monkey 39-week toxicity study, this effect was observed at doses of 1.5 mg/kg (approximately equal to the MRHD) and above and demonstrated evidence of reversibility after cessation of treatment. The significance of this finding in humans is unknown.
14. Clinical Studies
14.1 Heavy Menstrual Bleeding Associated with Uterine Fibroids
The efficacy and safety of MYFEMBREE were evaluated in two replicate, 24-week, multinational, randomized, double-blind, placebo-controlled studies in a total of 768 premenopausal women with heavy menstrual bleeding associated with uterine fibroids in Study L1 (NCT03049735) and Study L2 (NCT03103087).
For study inclusion, women had to have uterine fibroids confirmed by ultrasound examination in which at least one fibroid met at least one of the following criteria:
- a.
- Subserosal, intramural, or < 50% intracavitary submucosal fibroid with a diameter ≥ 2 cm, or
- b.
- Multiple small fibroids with a total uterine volume of ≥ 130cm3.
Women also had to have menstrual blood loss (MBL) volume of ≥ 80 mL per cycle for two menstrual cycles or ≥ 160 mL during one cycle quantified by the alkaline hematin method from menstrual products collected during baseline menstrual cycles to be included in the studies. Women with hemoglobin < 8.0 g/dL were excluded from the study. Iron therapy was required for women with hemoglobin ≥ 8 g/dL and ≤ 10 g/dL. Women were allowed, but not required, to take calcium and vitamin D during the study.
In Studies L1 and L2, women were randomized 1:1:1 to receive a once daily relugolix 40 mg tablet plus an over encapsulated tablet of E2 1 mg and NETA 0.5 mg (relugolix+E2/NETA), which is equivalent to 1 tablet of MYFEMBREE, for 24 weeks, placebo for 24 weeks, or relugolix 40 mg monotherapy for 12 weeks followed by MYFEMBREE for 12 weeks. Treatment was initiated within the first seven days after the onset of menses.
The primary endpoint was the proportion of women in the MYFEMBREE group compared with women in the placebo group, who achieved menstrual blood loss volume of < 80 mL and at least a 50% reduction from baseline MBL volume over the last 35 days of treatment, as measured by the alkaline hematin method. Key secondary endpoints were related to amenorrhea, MBL volume, and change in hemoglobin.
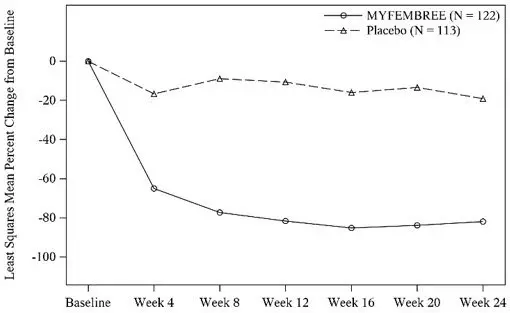
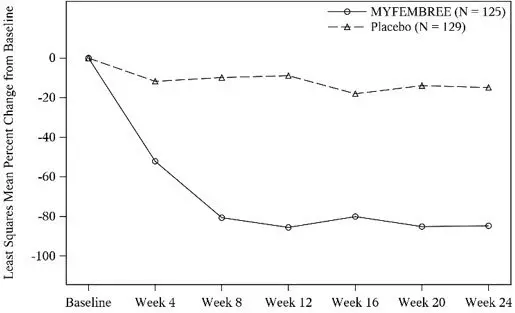
A total of 768 women were randomized and treated in Studies L1 and L2 (741 women in the efficacy population used for these studies). Of the 741 women, 247 received treatment with MYFEMBREE (122 and 125 in Studies L1 and L2, respectively), 252 received treatment with relugolix followed by MYFEMBREE (125 and 127 in Studies L1 and L2, respectively), and 242 received placebo (113 and 129 in Studies L1 and L2, respectively). The median age of women included in the efficacy analysis was 43 years (19 - 51 years), and mean body mass index was 31.6 kg/m2. Approximately 53% of study participants were Black, 41% were White, and 6% were of other races. Across studies at baseline, mean (± standard deviation) MBL volume at baseline was 231 mL (± 156). Baseline uterine size in Studies L1 and L2 ranged from normal to greater than 28 weeks gestation size (47 - 2625 cm3).
Hemoglobin Levels
For efficacy, a hemoglobin response was defined as a hemoglobin increase > 2 g/dL from baseline to Week 24 in the subgroup of women with anemia at baseline (hemoglobin ≤ 10.5 g/dL). A statistically higher proportion treated with MYFEMBREE compared with placebo had > 2 g/dL improvement in hemoglobin levels, see Table 9.
| Study L1 | Study L2 | |||
|---|---|---|---|---|
| MYFEMBREE n=43 (N = 122) | Placebo n=29 (N = 113) | MYFEMBREE n=40 (N = 125) | Placebo n=53 (N = 129) |
|
| Abbreviation: CI = confidence interval. | ||||
| n = number of patients with Hgb ≤10.5 g/dL at baseline. | ||||
| N = number of patients in each treatment group. | ||||
| % at Week 24 | 44.2% | 17.2% | 55.0% | 5.7% |
| Difference from placebo, % 95% CI p-value | 26.9% (6.7%, 47.2%) 0.0177 | 49.3% (32.7%, 66.0%) <0.0001 |
||
14.2 Moderate to Severe Pain Associated with Endometriosis
The efficacy of MYFEMBREE was assessed in two 24-week, multinational, randomized, double-blind, placebo-controlled studies in pre-menopausal women with moderate to severe pain associated with endometriosis in Study S1 (NCT03204318) and Study S2 (NCT03204331).
In Studies S1 and S2, women with moderate to severe pain associated with endometriosis were randomized 1:1:1 to receive once daily treatment with a tablet of relugolix 40 mg plus an over encapsulated tablet of E2 1 mg and NETA 0.5 mg, (equivalent to one tablet of MYFEMBREE) for 24 weeks, placebo for 24 weeks, or relugolix 40 mg monotherapy for 12 weeks followed by MYFEMBREE for 12 weeks.
For study inclusion, women had to have endometriosis confirmed by direct visualization during surgery and/or histology in addition to pain associated with endometriosis during a placebo run-in period. Dysmenorrhea (DYS) and non-menstrual pelvic pain (NMPP) were assessed daily using an 11-point numerical rating scale (NRS) ranging from 0 ("no pain") to 10 ("pain as bad as you can imagine"). Specifically, women had to have pain that met the following criteria:
- DYS NRS score ≥ 4.0 on at least 2 days AND
- Mean NMPP NRS score ≥ 2.5 or
Mean NMPP NRS score ≥ 1.25 and NMPP NRS score ≥ 5.0 on at least 4 days
Studies S1 and S2 each had two co-primary endpoints. The first co-primary endpoint was a responder analysis where a responder was defined as a woman who achieved a reduction from baseline in dysmenorrhea (DYS) NRS of at least 2.8 points over the last 35 days of treatment, without an increase in analgesic use (nonsteroidal anti-inflammatory drug or opioid). The second co-primary endpoint was a responder analysis where a responder was defined as a woman who achieved a reduction from baseline in non-menstrual pelvic pain (NMPP) NRS score of at least 2.1 points over the last 35 days of treatment, without an increase in analgesic use (nonsteroidal anti-inflammatory drug or opioid) for pain associated with endometriosis.
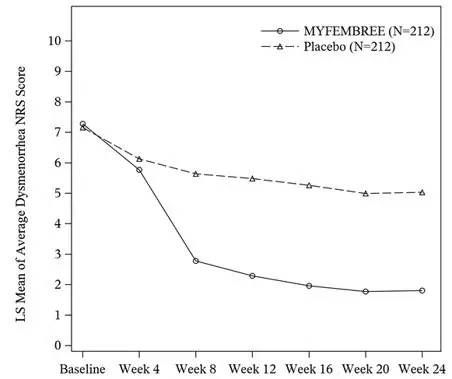
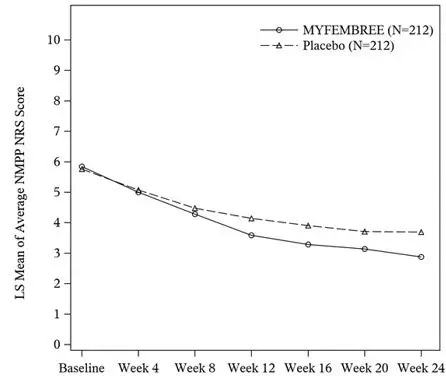
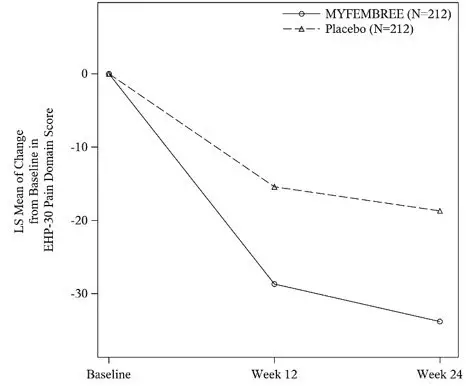
In Study S1, a total of 424 women were included in the efficacy population (212 received MYFEMBREE; 212 received placebo). The median age of the efficacy population was 34 years and the mean body mass index was 26 kg/m2. Approximately 92% were White, 6% were Black, and 7% were of Hispanic or Latino descent. A total of 19% were from the United States and/or Canada. At baseline, 29% of women used an opioid rescue analgesic for moderate to severe pain associated with endometriosis.
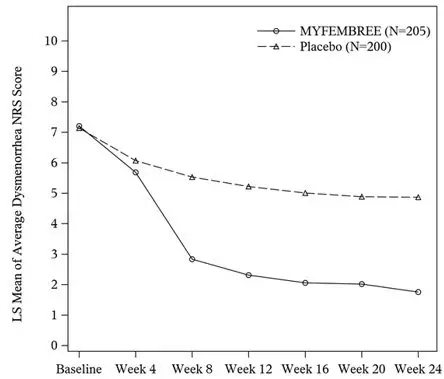
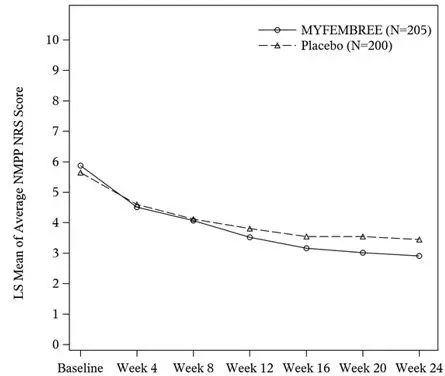
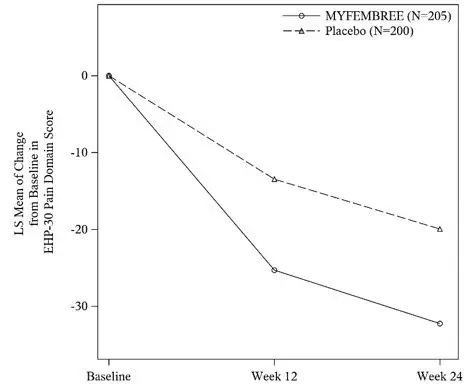
In Study S2, a total of 405 women were included in the efficacy population (205 received MYFEMBREE; 200 received placebo). The median age of the efficacy population was 34 years and the mean body mass index was 26 kg/m2. Approximately 90% were White, 6% were Black, and 17% were of Hispanic or Latino descent. A total of 24% were from United States and none were from Canada. At baseline, 48% of women used an opioid rescue analgesic for moderate to severe pain associated with endometriosis.
The results for the co-primary efficacy endpoints as assessed at Week 24 are shown below in Table 10.
| Study S1 | Study S2 | |||
|---|---|---|---|---|
| MYFEMBREE (N = 212) | Placebo (N = 212) | MYFEMBREE (N = 205) | Placebo (N = 200) |
|
| Abbreviations: CI = confidence interval. | ||||
| Responders are women with a reduction from baseline of at least 2.8 points on the NRS for dysmenorrhea or at least 2.1 points on the NRS for non-menstrual pelvic pain and no increase in analgesic use over the last 35 days of treatment. | ||||
| Dysmenorrhea | 74.5% | 26.9% | 75.1% | 30.5% |
| Difference from placebo (95% CI) p-value | 47.6% (39.3%, 56.0%) ≤ 0.0001 | - | 44.6% (35.9%, 53.3%) ≤ 0.0001 | - |
| Non-menstrual pelvic pain | 58.5% | 39.6% | 65.9% | 42.5% |
| Difference from placebo (95% CI) * p-value | 18.9% (9.5%, 28.2%) ≤ 0.0001 | - | 23.4% (13.9%, 32.8%) ≤ 0.0001 | - |
Key secondary efficacy endpoints included changes from baseline in the DYS NRS scores, NMPP NRS scores, Endometriosis Health Profile-30 (EHP-30) pain domain scores, dyspareunia NRS scores, and opioid use.
16. How is Myfembree supplied
16.1 How Supplied
MYFEMBREE film-coated tablets contain relugolix 40 mg, estradiol (E2) 1 mg, and norethindrone acetate (NETA) 0.5 mg. The tablets are light yellow to yellow, round film-coated tablet with "MVT" on one side and "415" on the other side.
MYFEMBREE is supplied in a white, opaque, high-density polyethylene bottle containing 28 tablets with desiccant and closed with an induction-sealed child-resistant cap (NDC Code 72974-415-01).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
| MYFEMBREE
relugolix, estradiol hemihydrate, and norethindrone acetate tablet, film coated |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Myovant Sciences, Inc. (080453011) |
| Registrant - Myovant Sciences GmbH (480146015) |