Drug Detail:Mylotarg (Gemtuzumab ozogamicin [ jem-tooz-ue-mab-oh-zoe-ga-mye-sin ])
Drug Class: CD33 monoclonal antibodies
Highlights of Prescribing Information
MYLOTARG™ (gemtuzumab ozogamicin) for injection, for intravenous use
Initial U.S. Approval: 2000
WARNING: HEPATOTOXICITY
See full prescribing information for complete boxed warning.
Hepatotoxicity, including severe or fatal hepatic veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), has been reported in association with the use of MYLOTARG. (5.1, 6.1)
Recent Major Changes
| Dosage and Adminstration, Instructions for Reconstitution, Dilution, and Administration (2.4) | 08/2021 |
Indications and Usage for Mylotarg
MYLOTARG is a CD33-directed antibody and cytotoxic drug conjugate indicated for:
- treatment of newly-diagnosed CD33-positive acute myeloid leukemia (AML) in adults and pediatric patients 1 month and older (1.1).
- treatment of relapsed or refractory CD33-positive AML in adults and pediatric patients 2 years and older (1.2).
Mylotarg Dosage and Administration
- Newly-diagnosed, de novo AML (combination regimen)
Adults:- –
- Induction: 3 mg/m2 (up to one 4.5 mg vial) on Days 1, 4, and 7 in combination with daunorubicin and cytarabine (2.2).
- –
- Consolidation: 3 mg/m2 on Day 1 (up to one 4.5 mg vial) in combination with daunorubicin and cytarabine (2.2).
- –
- 3 mg/m2 for patients with body surface area (BSA) 0.6 m2 or greater (2.2).
- –
- 0.1 mg/kg for patients with BSA less than 0.6 m2 (2.2).
- –
- See Full Prescribing Information for complete dosing information (2.2).
- Newly-diagnosed AML (single-agent regimen):
Adults:- –
- Induction: 6 mg/m2 (not limited to one 4.5 mg vial) on Day 1 and 3 mg/m2 (not limited to one 4.5 mg vial) on Day 8 (2.2).
- –
- Continuation: For patients without evidence of disease progression following induction, up to 8 continuation courses of MYLOTARG 2 mg/m2 (not limited to one 4.5 mg vial) on Day 1 every 4 weeks (2.2).
- Relapsed or refractory AML (single-agent regimen):
- Adults and pediatric patients 2 years and older:
- –
- 3 mg/m2 (up to one 4.5 mg vial) on Days 1, 4, and 7 (2.2).
- Premedicate with a corticosteroid, antihistamine, and acetaminophen (2.1).
Dosage Forms and Strengths
For Injection: 4.5 mg as a lyophilized cake or powder in a single-dose vial for reconstitution and dilution (3).
Contraindications
Hypersensitivity to MYLOTARG or any of its components (4).
Warnings and Precautions
- Infusion-related reactions (including anaphylaxis): Premedicate with a corticosteroid, acetaminophen, and diphenhydramine. Monitor patients during and for at least 1 hour after the end of the infusion. Interrupt the infusion, administer steroids or antihistamines, or permanently discontinue treatment as necessary (2.1, 5.2, 6).
- Hemorrhage: Severe, including fatal, hemorrhage may occur when MYLOTARG is used at recommended doses. Monitor platelet counts frequently (5.3, 6.1).
- Embryo-fetal toxicity: Can cause fetal harm. Advise patients of reproductive potential of the potential risk to a fetus and to use effective contraception (5.6, 8.1, 8.3).
Adverse Reactions/Side Effects
The most common adverse reactions (greater than 15%) were hemorrhage, infection, fever, nausea, vomiting, constipation, headache, increased AST, increased ALT, rash, mucositis, febrile neutropenia, and decreased appetite (6).
To report SUSPECTED ADVERSE REACTIONS, contact Pfizer Inc. at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Lactation: Advise not to breastfeed (8.2).
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 8/2021
Related/similar drugs
azacitidine, venetoclax, vincristine, Venclexta, cytarabine, ivosidenibFull Prescribing Information
WARNING: HEPATOTOXICITY
Hepatotoxicity, including severe or fatal hepatic veno-occlusive disease (VOD), also known as sinusoidal obstruction syndrome (SOS), has been reported in association with the use of MYLOTARG as a single agent, and as part of a combination chemotherapy regimen. Monitor frequently for signs and symptoms of VOD after treatment with MYLOTARG. (5.1 and 6.1)
1. Indications and Usage for Mylotarg
2. Mylotarg Dosage and Administration
2.1 Premedication and Special Considerations
- Premedicate adults with acetaminophen 650 mg orally and diphenhydramine 50 mg orally or intravenously 1 hour prior to MYLOTARG dosing and 1 mg/kg methylprednisolone or an equivalent dose of an alternative corticosteroid within 30 minutes prior to infusion of MYLOTARG.
- Premedicate pediatric patients 1 month and older with acetaminophen 15 mg/kg (maximum of 650 mg) and diphenhydramine 1 mg/kg (maximum of 50 mg) 1 hour prior to MYLOTARG dosing, and 1 mg/kg methylprednisolone orally or intravenously within 30 minutes prior to infusion of MYLOTARG; additional doses of acetaminophen and diphenhydramine may be administered every 4 hours after the initial pretreatment dose. Repeat with the same dose of methylprednisolone or an equivalent corticosteroid for any sign of an infusion reaction, such as fever, chills, hypotension, or dyspnea during the infusion or within 4 hours afterwards [see Warnings and Precautions (5.2)].
- Use appropriate measures to prevent tumor lysis syndrome.
- For patients with hyperleukocytosis (leukocyte count greater than or equal to 30 Gi/L), cytoreduction is recommended prior to administration of MYLOTARG.
2.3 Dosage Modifications for Toxicities
Monitor blood counts frequently through resolution of cytopenias. Monitor blood counts and chemistries at least three times per week through recovery from treatment-related toxicities. Management of some adverse reactions [see Warnings and Precautions (5), Adverse Reactions (6)] may require dose interruptions or permanent discontinuation of MYLOTARG. Table 1 shows the dose modification guidelines for hematologic and nonhematologic toxicities.
| Hematologic and Nonhematologic Toxicities | Recommended Action |
|---|---|
| Abbreviations: ALT=alanine aminotransferase; AST=aspartate aminotransferase; VOD=veno-occlusive disease; ULN=upper limit of normal. | |
| For patients receiving MYLOTARG in combination therapy | |
| Persistent thrombocytopenia |
|
| Persistent neutropenia |
|
| For all patients receiving MYLOTARG (Monotherapy or in Combination) | |
| VOD |
|
| Total bilirubin greater than 2 × ULN, or AST and/or ALT greater than 2.5 × ULN |
|
| Infusion-related reactions |
|
| Other severe or life-threatening non-hematologic toxicities |
|
3. Dosage Forms and Strengths
For injection: 4.5 mg as a white to off-white lyophilized cake or powder in a single-dose vial for reconstitution and further dilution.
4. Contraindications
MYLOTARG is contraindicated in patients with a history of hypersensitivity to the active substance in MYLOTARG or any of its components or to any of the excipients. Reactions have included anaphylaxis [see Warnings and Precautions (5.2), Adverse Reactions (6)].
5. Warnings and Precautions
5.1 Hepatotoxicity, Including Veno-occlusive Liver Disease (VOD)
Hepatotoxicity, including life-threatening and sometimes fatal hepatic VOD events, have been reported in patients receiving MYLOTARG as a single agent or as part of a combination chemotherapy regimen [see Adverse Reactions (6)].
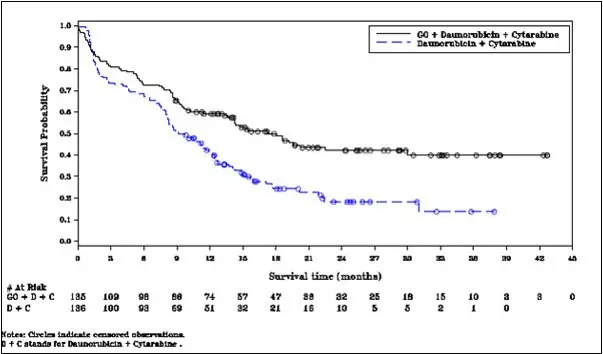
In ALFA-0701, VOD events were reported in 6/131 (5%) adult patients during or following treatment with MYLOTARG, or following later hematopoietic stem cell transplantation (HSCT). The median time from the MYLOTARG dose to onset of VOD was 9 days (range: 2–298 days), with 5 events occurring within 28 days of any dose of MYLOTARG and 1 event occurring greater than 28 days after the last dose of MYLOTARG. Three of the 6 VOD events were fatal. VOD was also reported in 2 patients in the control arm of ALFA-0701 after receiving MYLOTARG as a therapy for relapsed AML.
In MyloFrance-1 (MYLOTARG 3 mg/m2 on Days 1, 4 and 7), VOD events were reported in none of the 57 patients during or following treatment, or following HSCT after completion of MYLOTARG treatment.
In AAML0531, VOD events were reported in 25/520 (5%) pediatric patients in the MYLOTARG arm. VOD was fatal in 2 patients. Among 187 pediatric patients who underwent HSCT in the MYLOTARG arm, VOD occurred within 30 days post-HSCT in 20 (11%) patients.
Based on an analysis across trials, the risk of VOD was higher in adult patients who received higher doses of MYLOTARG as monotherapy, in patients with moderate or severe hepatic impairment prior to receiving MYLOTARG, in patients treated with MYLOTARG after HSCT, and in patients who underwent HSCT after treatment with MYLOTARG. Patients who had moderate/severe hepatic impairment prior to treatment with MYLOTARG were 8.7 times more likely to develop VOD compared to patients without moderate/severe hepatic impairment at baseline. Patients treated with MYLOTARG for relapse after HSCT were 2.6 times more likely to develop VOD compared to patients without prior HSCT. Patients who underwent HSCT following MYLOTARG treatment were 2.9 times more likely to develop VOD after HSCT compared to patients without HSCT following MYLOTARG treatment. Although no relationship was found between VOD and time of HSCT relative to higher MYLOTARG monotherapy doses, the ALFA-0701 study recommended an interval of 2 months between the last dose of MYLOTARG and HSCT. In MyloFrance-1, no patients underwent HSCT within 3.5 months of MYLOTARG therapy.
Assess ALT, AST, total bilirubin, and alkaline phosphatase prior to each dose of MYLOTARG. After treatment with MYLOTARG, monitor frequently for signs and symptoms of VOD; these may include elevations in ALT, AST, total bilirubin, hepatomegaly (which may be painful), rapid weight gain, and ascites. Monitoring only total bilirubin may not identify all patients at risk of VOD. For patients who develop abnormal liver tests, more frequent monitoring of liver tests and clinical signs and symptoms of hepatotoxicity is recommended. For patients who proceed to HSCT, monitor liver tests frequently during the post-HSCT period, as appropriate.
Manage signs or symptoms of hepatic toxicity by dose interruption or discontinuation of MYLOTARG [see Dosage and Administration (2.3)]. In patients who experience VOD, discontinue MYLOTARG and treat according to standard medical practice.
5.2 Infusion-Related Reactions (Including Anaphylaxis)
Life-threatening or fatal infusion-related-reactions can occur during or within 24 hours following infusion of MYLOTARG [see Adverse Reactions (6)]. Signs and symptoms of infusion-related reactions may include fever, chills, hypotension, tachycardia, hypoxia and respiratory failure.
Premedicate prior to MYLOTARG infusion [see Dosage and Administration (2.1)]. Monitor vital signs frequently during infusion. Interrupt infusion immediately for patients who develop evidence of infusion reaction, especially dyspnea, bronchospasm, or hypotension. Monitor patients during and for at least 1 hour after the end of the infusion or until signs and symptoms completely resolve. Discontinue use of MYLOTARG in patients who develop signs or symptoms of anaphylaxis, including severe respiratory symptoms or clinically significant hypotension [see Dosage and Administration (2.2)].
5.3 Hemorrhage
MYLOTARG is myelosuppressive and can cause fatal or life-threatening hemorrhage due to prolonged thrombocytopenia. In ALFA-0701, (MYLOTARG in combination with chemotherapy), all grades and Grade 3–4 bleeding events were reported in 118/131 (90%) and 27/131 (21%) patients, respectively. Fatal bleeding events (including cerebral hematoma, intracranial hematoma, and subdural hematoma) occurred in 4/131 (3%) patients. Thrombocytopenia with platelet counts less than 50 Gi/L persisting more than 42 days occurred in 19 (19%) patients in the induction phase [see Adverse Reactions (6)]. The proportion of patients with persistent thrombocytopenia increased with progressive treatment phases and was higher in patients treated with MYLOTARG plus chemotherapy than with chemotherapy alone [see Adverse Reactions (6)].
In AAML0531, fatal bleeding occurred in 3/520 (<1%) of the pediatric patients. Grade 3 or 4 bleeding was reported in 66/520 (13%) of the pediatric patients in the MYLOTARG arm.
In AML-19 (MYLOTARG monotherapy at 6 mg/m2 Day 1 and 3 mg/m2 Day 8), all grades and Grade 3 or higher bleeding were reported in 28/111 (25%) and 14/111 (13%) patients, respectively. Fatal bleeding occurred in 1/111 (1%). In MyloFrance-1 (MYLOTARG 3 mg/m2 as monotherapy), Grade 3 bleeding was reported in 4/57 (7%) patients, but no patient experienced Grade 4 hemorrhage.
Assess blood counts prior to each dose of MYLOTARG and monitor blood counts frequently after treatment with MYLOTARG until resolution of cytopenias. Monitor patients for signs and symptoms of bleeding during treatment with MYLOTARG. Manage severe bleeding, hemorrhage or persistent thrombocytopenia using dose delay or permanent discontinuation of MYLOTARG [see Dosage and Administration (2.2)], and provide supportive care per standard practice.
5.4 QT Interval Prolongation
QT interval prolongation has been observed in patients treated with other drugs containing calicheamicin. When administering MYLOTARG to patients who have a history of or predisposition for QTc prolongation, who are taking medicinal products that are known to prolong QT interval, and in patients with electrolyte disturbances, obtain electrocardiograms (ECGs) and electrolytes prior to the start of treatment and as needed during administration.
5.5 Use in AML with Adverse-Risk Cytogenetics
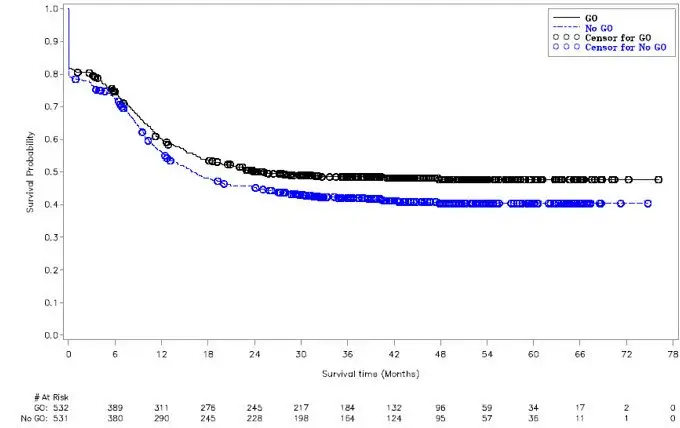
In subgroup analyses in ALFA-0701, the addition of MYLOTARG to standard combination chemotherapy did not improve event-free survival in the subgroup of patients having adverse-risk cytogenetics (HR 1.11; 95% CI: 0.63, 1.95). For patients being treated with MYLOTARG in combination with daunorubicin and cytarabine for newly-diagnosed de novo AML, when cytogenetics testing results become available consider whether the potential benefit of continuing treatment with MYLOTARG outweighs the risks for the individual patient.
5.6 Embryo-Fetal Toxicity
Based on its mechanism of action and findings from animal studies, MYLOTARG can cause embryo-fetal harm when administered to a pregnant woman. In animal studies, gemtuzumab ozogamicin caused embryo-fetal toxicity, starting at a dose that was approximately 0.4 times the exposure in patients at the maximum recommended dose, based on the area under the concentration-time curve (AUC).
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with MYLOTARG and for at least 6 months after the final dose. Advise males with female partners of reproductive potential to use effective contraception during treatment with MYLOTARG and for at least 3 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Hepatotoxicity, including VOD [see Warnings and Precautions (5.1)]
- Infusion-related reactions [see Warnings and Precautions (5.2)]
- Hemorrhage [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
6.2 Postmarketing Experience
The following adverse drug reactions have been identified during post-approval use of MYLOTARG. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: Neutropenic colitis1
Infections and Infestations: fungal lung infections including Pulmonary mycosis and Pneumocystis jirovecii pneumonia1; and bacterial infections including Stenotrophomonas infection
Renal and Urinary Disorders: Hemorrhagic cystitis1
Respiratory, Thoracic and Mediastinal Disorders: Interstitial pneumonia1
- 1
- including fatal events
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
MYLOTARG can cause embryo-fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
The safety and effectiveness of MYLOTARG in combination with standard chemotherapy have been established in pediatric patients 1 month and older with newly-diagnosed de novo AML. The use of MYLOTARG for this indication is supported by evidence of effectiveness from adequate and well-controlled studies in adults with supportive data on safety and effectiveness in Study AAML0531 (NCT00372593) [see Adverse Reactions (6.1), Clinical Studies (14.1)]. AAML0531 included patients in the following age groups: 2 patients less than 27 days old, 94 patients 28 days to less than 2 years old, 225 patients 2 years to less than 12 years old, 175 patients 12 years old to less than 18 years old, and 36 patients 18 years or older in the MYLOTARG plus chemotherapy arm. The safety and effectiveness of MYLOTARG with standard chemotherapy in pediatric patients less than 1 month of age with newly-diagnosed de novo AML have not been established.
The safety and effectiveness of MYLOTARG as a single agent in pediatric patients with newly-diagnosed AML have not been established.
The safety and effectiveness of MYLOTARG as a single agent in the pediatric patients with relapsed or refractory AML is supported by a single-arm trial in 29 patients in the following age groups: 1 patient 1 month to less than 2 years old, 13 patients 2 years to less than 12 years old, and 15 patients 12 years to 18 years old. A literature review included an additional 96 patients with ages ranging from 0.2 to 21 years. No differences in efficacy and safety were observed by age. The information on this use is discussed throughout the labeling. The safety and effectiveness of MYLOTARG as a single agent in pediatric patients less than 2 years of age with relapsed or refractory AML have not been established.
8.5 Geriatric Use
Use of MYLOTARG in combination with daunorubicin and cytarabine in newly-diagnosed adult patients with de novo AML is supported by a randomized, controlled trial that included 50 patients greater than or equal to 65 years old. No overall differences in safety or effectiveness were observed between these subjects and younger subjects. Use of MYLOTARG monotherapy in newly-diagnosed adult patients with AML is supported by a randomized controlled trial with 118 patients treated with MYLOTARG. All patients were over the age of 60 years and 65% of patients were above 75 years. No overall differences in effectiveness were observed by age.
Use of MYLOTARG as single-agent treatment of relapsed or refractory AML is supported by a single-arm trial that included 27 patients 65 years or older. No overall differences in effectiveness were observed between these patients and younger patients. Elderly patients experienced a higher rate of fever and severe or greater infections.
11. Mylotarg Description
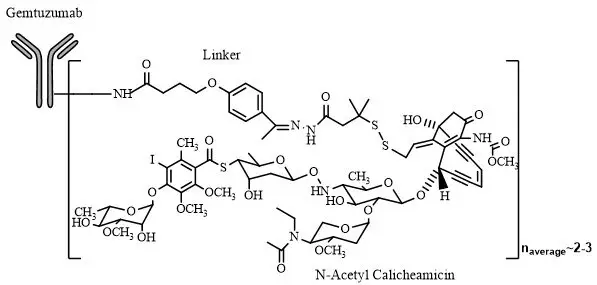
Gemtuzumab ozogamicin is an antibody-drug conjugate (ADC) composed of the CD33-directed monoclonal antibody (hP67.6; recombinant humanized immunoglobulin [Ig] G4, kappa antibody produced by mammalian cell culture in NS0 cells) that is covalently linked to the cytotoxic agent N-acetyl gamma calicheamicin. Gemtuzumab ozogamicin consists of conjugated and unconjugated gemtuzumab. The conjugated molecules differ in the number of activated calicheamicin derivative moieties attached to gemtuzumab. The number of conjugated calicheamicin derivatives per gemtuzumab molecule ranges from predominantly zero to 6, with an average of 2 to 3 moles of calicheamicin derivative per mole of gemtuzumab.
MYLOTARG (gemtuzumab ozogamicin) for injection is supplied as a sterile, white to off-white, preservative-free lyophilized cake or powder for intravenous administration. Each single-dose vial delivers 4.5 mg gemtuzumab ozogamicin. Inactive ingredients are dextran 40 (41.0 mg), sodium chloride (26.1 mg), sodium phosphate dibasic anhydrous (2.7 mg), sodium phosphate monobasic monohydrate (0.45 mg), and sucrose (69.8 mg). After reconstitution with 5 mL of Sterile Water for Injection USP, the concentration is 1 mg/mL of gemtuzumab ozogamicin with a deliverable volume of 4.5 mL (4.5 mg).
12. Mylotarg - Clinical Pharmacology
12.1 Mechanism of Action
Gemtuzumab ozogamicin is a CD33-directed antibody-drug conjugate (ADC). The antibody portion (hP67.6) recognizes human CD33 antigen. The small molecule, N-acetyl gamma calicheamicin, is a cytotoxic agent that is covalently attached to the antibody via a linker. Nonclinical data suggest that the anticancer activity of gemtuzumab ozogamicin is due to the binding of the ADC to CD33-expressing tumor cells, followed by internalization of the ADC-CD33 complex, and the intracellular release of N-acetyl gamma calicheamicin dimethyl hydrazide via hydrolytic cleavage of the linker. Activation of N-acetyl gamma calicheamicin dimethyl hydrazide induces double-strand DNA breaks, subsequently inducing cell cycle arrest and apoptotic cell death.
12.2 Pharmacodynamics
Saturation of a high percentage of CD33 antigenic sites is presumed to be required for maximum delivery of calicheamicin to leukemic blast cells. Near maximal peripheral CD33 saturation was observed across studies after gemtuzumab ozogamicin dosing at dose levels of 2 mg/m2 and above.
At 9 mg/m2 gemtuzumab ozogamicin (2 doses, 14 days apart), the risk for VOD increases as the Cmax of the first dose of gemtuzumab ozogamicin increases. The increase in VOD is more prominent in patients with prior stem cell transplantation.
12.3 Pharmacokinetics
There are no clinical PK data for the fractionated regimen. When gemtuzumab ozogamicin is administered at 9 mg/m2 (2 doses, 14 days apart), the Cmax following the first dose for patients who received 9 mg/m2 gemtuzumab ozogamicin was 3.0 mg/L and increased to 3.6 mg/L after the second dose.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Formal carcinogenicity studies have not been conducted with gemtuzumab ozogamicin. In toxicity studies, rats were dosed weekly for 6 weeks with gemtuzumab ozogamicin at doses up to 7.2 mg/m2/week. After 6 weeks of dosing, rats developed oval cell hyperplasia in the liver, which is considered a potentially preneoplastic finding, at 7.2 mg/m2/week (approximately 16 times the exposure in patients at the maximum recommended dose, based on AUC). Other preneoplastic or neoplastic changes observed with other antibody-calicheamicin conjugates in rats included basophilic and/or eosinophilic altered cell foci and hepatocellular adenomas. The relevance of these animal findings to humans is uncertain.
Gemtuzumab ozogamicin was clastogenic in vivo in the bone marrow of mice that received single doses greater than or equal to 22.1 mg/m2. This is consistent with the known induction of DNA breaks by calicheamicin. N-acetyl gamma calicheamicin dimethyl hydrazide (the released cytotoxic agent) was mutagenic in the bacterial reverse mutation assay and clastogenic in the in vitro micronucleus assay in human TK6 cells.
In a female fertility study, female rats were administered daily intravenous doses of gemtuzumab ozogamicin up to 1.08 mg/m2 for 14 days before mating with untreated male rats. Significant decreases in the numbers of corpora lutea and implants were observed at 1.08 mg/m2, and dose-related decreases and increases in the number of live and dead embryos were observed at doses tested (approximately 0.4 times the exposure in patients at the maximum recommended dose, based on AUC). Increased embryofetal lethality at ≥0.36 mg/m2 was observed in the presence of maternal toxicity that included decreases in gestational body weight and food consumption. Additional findings in female reproductive organs (ovarian atrophy and decreased numbers of follicles associated with atrophy of the uterus, vagina and mammary glands) occurred in rats and monkeys after dosing with other antibody-calicheamicin conjugates.
Fertility was assessed in male rats administered daily intravenous doses of gemtuzumab ozogamicin from 0.12 to 1.08 mg/m2 for 28 days, followed by mating with untreated females, either at the end of the dosing period or after a 9-week drug-free period. Male fertility index was decreased at doses ≥0.12 mg/m2 (approximately 1.2 times the exposure in patients at the maximum recommended dose, based on AUC). Effects on testes and epididymides occurred at ≥0.12 mg/m2, including smaller size and lower weights in addition to adverse effects on sperm. Partial recovery was noted for some effects. Additional effects in male reproductive organs occurred in repeat-dose toxicology studies and included effects on mammary gland, testes, and epididymides in rats at ≥2.4 mg/m2/week and effects on testes and epididymides in monkeys at 21.6 mg/m2/week. Testicular effects in male monkeys with other antibody-calicheamicin conjugates included degeneration of seminiferous tubules and decreased epididymidal sperm, which did not reverse following a 6-week drug-free period.
14. Clinical Studies
16. How is Mylotarg supplied
MYLOTARG (gemtuzumab ozogamicin) for injection is a white to off-white lyophilized cake or powder supplied in a carton (NDC 0008-4510-01) containing one 4.5 mg single-dose vial [see Dosage and Administration (2)].
| MYLOTARG
gemtuzumab ozogamicin injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc. (113008515) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia & Upjohn Company LLC | 618054084 | PACK(0008-4510) , LABEL(0008-4510) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Wyeth Pharmaceutical Division of Wyeth Holdings LLC | 054065909 | ANALYSIS(0008-4510) , MANUFACTURE(0008-4510) , API MANUFACTURE(0008-4510) | |