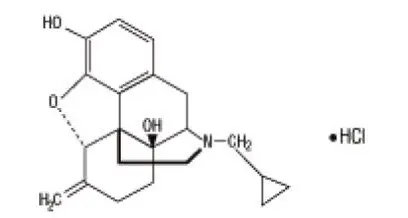
Drug Detail:Nalmefene (monograph) (Medically reviewed)
Drug Class:
Nalmefene Hydrochloride Injection - Clinical Pharmacology
Pharmacokinetics
Nalmefene exhibited dose proportional pharmacokinetics following intravenous administration of 0.5 mg to 2 mg. Pharmacokinetic parameters for nalmefene after a 1 mg intravenous administration in adult male volunteers are listed in Table 1.
| Parameter | Young, N=18 | Elderly, N=11 |
|---|---|---|
| Age | 19 to 32 | 62 to 80 |
| Cp at 5 min. (ng/mL) | 3.7 (29) | 5.8 (38) |
| Vdss (L/kg) | 8.6 (19) | 8.6 (29) |
| Vc (L/kg) | 3.9 (29) | 2.8 (41) |
| AUC0-inf (ng-hr/mL) | 16.6 (27) | 17.3 (14) |
| Terminal T1/2 (hr) | 10.8 (48) | 9.4 (49) |
| Clplasma (L/hr/kg) | 0.8 (23) | 0.8 (18) |
Adverse Reactions/Side Effects
Adverse event information was obtained following administration of nalmefene hydrochloride injection to 152 normal volunteers and in controlled clinical trials to 1127 patients for the treatment of opioid overdose or for postoperative opioid reversal.
Nalmefene was well tolerated and showed no serious toxicity during experimental administration to healthy individuals, even when given at 15 times the highest recommended dose. In a small number of subjects, at doses exceeding the recommended nalmefene hydrochloride injection dose, nalmefene produced symptoms suggestive of reversal of endogenous opioids, such as have been reported for other narcotic antagonist drugs. These symptoms (nausea, chills, myalgia, dysphoria, abdominal cramps, and joint pain) were usually transient and occurred at very low frequency.
Such symptoms of precipitated opioid withdrawal at the recommended clinical doses were seen in both postoperative and overdose patients who were later found to have had histories of covert opioid use. Symptoms of precipitated withdrawal were similar to those seen with other opioid antagonists, were transient following the lower doses used in the postoperative setting, and more prolonged following the administration of the larger doses used in the treatment of overdose.
Tachycardia and nausea following the use of nalmefene in the postoperative setting were reported at the same frequencies as for naloxone at equivalent doses. The risk of both these adverse events was low at doses giving partial opioid reversal and increased with increases in dose. Thus, total doses larger than 1 mcg/kg in the postoperative setting and 1.5 mg/70 kg in the treatment of overdose are not recommended.
| Adverse Event | Nalmefene N=1127 | Naloxone N=369 | Placebo N=77 |
|---|---|---|---|
| Nausea | 18% | 18% | 6% |
| Vomiting | 9% | 7% | 4% |
| Tachycardia | 5% | 8% | - |
| Hypertension | 5% | 7% | - |
| Postoperative pain | 4% | 4% | N/A |
| Fever | 3% | 4% | - |
| Dizziness | 3% | 4% | 1% |
| Headache | 1% | 1% | 4% |
| Chills | 1% | 1% | - |
| Hypotension | 1% | 1% | - |
| Vasodilation | 1% | 1% | - |
| NALMEFENE HYDROCHLORIDE
nalmefene hydrochloride injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Purdue Pharma L.P. (932323652) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Ajinomoto Althea, Inc. | 023050730 | MANUFACTURE(59011-960) | |