Drug Detail:Namenda xr (Memantine [ meh-man-teen ])
Drug Class: Miscellaneous central nervous system agents
Highlights of Prescribing Information
NAMENDA XR® (memantine hydrochloride) extended-release capsules, for oral use
Initial U.S. Approval: 2003
Indications and Usage for Namenda XR
NAMENDA XR is a N-methyl-D-aspartate (NMDA) receptor antagonist indicated for the treatment of moderate to severe dementia of the Alzheimer’s type. (1)
Namenda XR Dosage and Administration
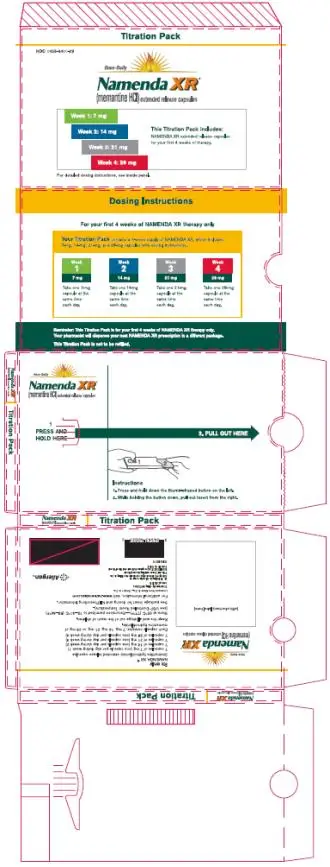
- The recommended starting dose of NAMENDA XR is 7 mg once daily; the dose should be increased in 7 mg increments to the recommended maintenance dose of 28 mg once daily; the minimum recommended interval between dose increases is one week. (2.1)
- Patients with severe renal impairment: the recommended maintenance dose of NAMENDA XR is 14 mg once daily. (2.3)
Dosage Forms and Strengths
- NAMENDA XR is available as an extended-release capsule in the following strengths: 7 mg, 14 mg, 21 mg, 28 mg (3)
Contraindications
- NAMENDA XR is contraindicated in patients with known hypersensitivity to memantine hydrochloride or to any excipients used in the formulation. (4)
Warnings and Precautions
- Conditions that raise urine pH may decrease the urinary elimination of memantine resulting in increased plasma levels of memantine. (5.1, 7.1)
Adverse Reactions/Side Effects
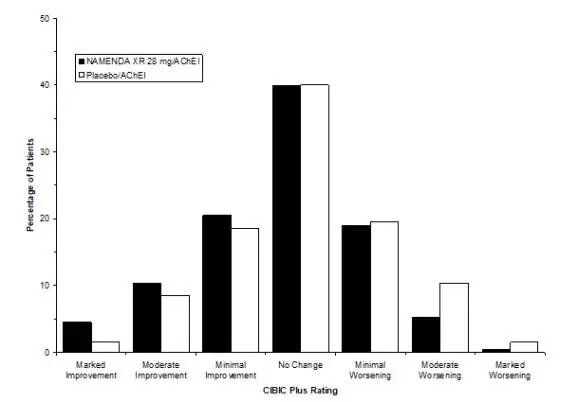
The most commonly observed adverse reactions occurring at a frequency of at least 5% and greater than placebo with administration of NAMENDA XR 28 mg/day were headache, diarrhea and dizziness. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Allergan at 1-800-678-1605 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
Related/similar drugs
donepezil, memantine, Aricept, Namenda, rivastigmine, ExelonFull Prescribing Information
1. Indications and Usage for Namenda XR
NAMENDA XR® is indicated for the treatment of moderate to severe dementia of the Alzheimer’s type.
2. Namenda XR Dosage and Administration
2.1 Recommended Dosing
The dosage of NAMENDA XR shown to be effective in a controlled clinical trial is 28 mg once daily.
The recommended starting dose of NAMENDA XR is 7 mg once daily. The dose should be increased in 7 mg increments to the recommended maintenance dose of 28 mg once daily. The minimum recommended interval between dose increases is one week. The dose should only be increased if the previous dose has been well tolerated. The maximum recommended dose is 28 mg once daily.
NAMENDA XR can be taken with or without food. NAMENDA XR capsules can be taken intact or may be opened, sprinkled on applesauce, and thereby swallowed. The entire contents of each NAMENDA XR capsule should be consumed; the dose should not be divided.
Except when opened and sprinkled on applesauce, as described above, NAMENDA XR should be swallowed whole. NAMENDA XR capsules should not be divided, chewed, or crushed.
If a patient misses a single dose of NAMENDA XR, that patient should not double up on the next dose. The next dose should be taken as scheduled. If a patient fails to take NAMENDA XR for several days, dosing may need to be resumed at lower doses and retitrated as described above.
3. Dosage Forms and Strengths
Each capsule contains 7 mg, 14 mg, 21 mg, or 28 mg of memantine hydrochloride.
- The 7 mg capsules are a yellow opaque capsule, with “FLI 7 mg” black imprint.
- The 14 mg capsules are a yellow cap and dark green opaque body capsule, with “FLI 14 mg” black imprint on the yellow cap.
- The 21 mg capsules are a white to off-white cap and dark green opaque body capsule, with “FLI 21 mg” black imprint on the white to off-white cap.
- The 28 mg capsules are a dark green opaque capsule, with “FLI 28 mg” white imprint.
4. Contraindications
NAMENDA XR is contraindicated in patients with known hypersensitivity to memantine hydrochloride or to any excipients used in the formulation.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
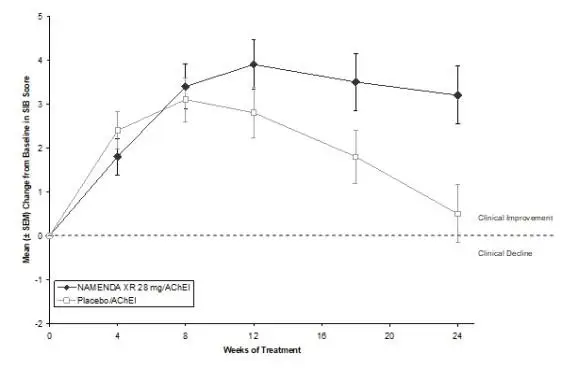
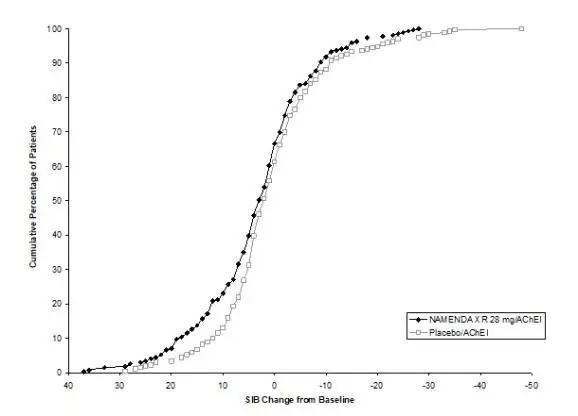
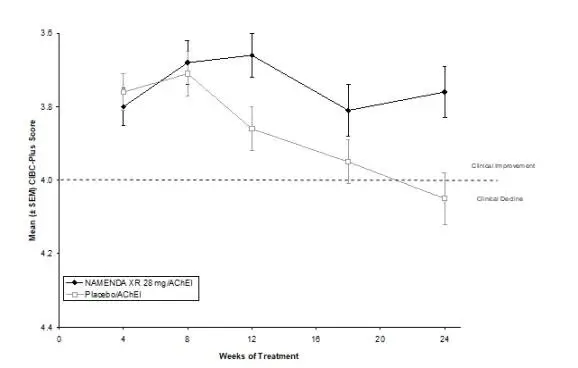
NAMENDA XR was evaluated in a double-blind placebo-controlled trial in which a total of 676 patients with moderate to severe dementia of the Alzheimer’s type (341 patients on NAMENDA XR 28 mg/day and 335 patients on placebo) were treated for up to 24 weeks.
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions Leading to Discontinuation
In the placebo-controlled clinical trial of NAMENDA XR, the proportion of patients in the NAMENDA XR group and the placebo group who discontinued treatment due to adverse reactions was 10% and 6%, respectively. The most common adverse reaction that led to treatment discontinuation in the NAMENDA XR group was dizziness, at a rate of 1.5%.
Most Common Adverse Reactions
The most commonly observed adverse reactions seen in patients administered NAMENDA XR in the controlled clinical trial, defined as those occurring at a frequency of at least 5% in the NAMENDA XR group and at a frequency higher than placebo, were headache, diarrhea and dizziness.
Table 1 lists adverse reactions that were observed at an incidence of ≥ 2% in the NAMENDA XR group and occurred at a rate greater than placebo.
| Adverse Reaction | Placebo
(n=335) % | NAMENDA XR 28 mg
(n=341) % |
| Gastrointestinal Disorders | ||
| Diarrhea | 4 | 5 |
| Constipation | 1 | 3 |
| Abdominal pain | 1 | 2 |
| Vomiting | 1 | 2 |
| Infections and Infestations | ||
| Influenza | 3 | 4 |
| Investigations | ||
| Weight, increased | 1 | 3 |
| Musculoskeletal and Connective Tissue Disorders | ||
| Back pain | 1 | 3 |
| Nervous System Disorders | ||
| Headache | 5 | 6 |
| Dizziness | 1 | 5 |
| Somnolence | 1 | 3 |
| Psychiatric Disorders | ||
| Anxiety | 3 | 4 |
| Depression | 1 | 3 |
| Aggression | 1 | 2 |
| Renal and Urinary Disorders | ||
| Urinary incontinence | 1 | 2 |
| Vascular Disorders | ||
| Hypertension | 2 | 4 |
| Hypotension | 1 | 2 |
Seizure
Memantine has not been systematically evaluated in patients with a seizure disorder. In clinical trials of memantine, seizures occurred in 0.3% of patients treated with memantine and 0.6% of patients treated with placebo.
7. Drug Interactions
7.1 Drugs That Make Urine Alkaline
The clearance of memantine was reduced by about 80% under alkaline urine conditions at pH 8. Therefore, alterations of urine pH towards the alkaline condition may lead to an accumulation of the drug with a possible increase in adverse effects. Urine pH is altered by diet, drugs (e.g. carbonic anhydrase inhibitors, sodium bicarbonate) and clinical state of the patient (e.g. renal tubular acidosis or severe infections of the urinary tract). Hence, memantine should be used with caution under these conditions.
12. Namenda XR - Clinical Pharmacology
12.3 Pharmacokinetics
Memantine is well absorbed after oral administration and has linear pharmacokinetics over the therapeutic dose range. It is excreted predominantly unchanged in urine and has a terminal elimination half-life of about 60-80 hours. In a study comparing 28 mg once daily NAMENDA XR to 10 mg twice daily NAMENDA, the Cmax and AUC0-24 values were 48% and 33% higher for the XR dosage regimen, respectively.
Absorption
After multiple dose administration of NAMENDA XR, memantine peak concentrations occur around 9-12 hours post-dose. There is no difference in the absorption of NAMENDA XR when the capsule is taken intact or when the contents are sprinkled on applesauce.
There is no difference in memantine exposure, based on Cmax or AUC, for NAMENDA XR whether that drug product is administered with food or on an empty stomach. However, peak plasma concentrations are achieved about 18 hours after administration with food versus approximately 25 hours after administration on an empty stomach.
Distribution
The mean volume of distribution of memantine is 9-11 L/kg and the plasma protein binding is low (45%).
Elimination
Metabolism
Memantine undergoes partial hepatic metabolism. The hepatic microsomal CYP450 enzyme system does not play a significant role in the metabolism of memantine.
Excretion
Memantine is excreted predominantly unchanged in the urine and has a terminal elimination half-life of about 60-80 hours. About 48% of administered drug is excreted unchanged in urine; the remainder is converted primarily to three polar metabolites which possess minimal NMDA receptor antagonistic activity: the N-glucuronide conjugate, 6-hydroxy-memantine, and 1-nitroso-deaminated memantine. A total of 74% of the administered dose is excreted as the sum of the parent drug and the N-glucuronide conjugate. Renal clearance involves active tubular secretion moderated by pH dependent tubular reabsorption.
Specific Populations
Elderly
The pharmacokinetics of memantine in young and elderly subjects are similar.
Gender
Following multiple dose administration of memantine hydrochloride 20 mg daily, females had about 45% higher exposure than males, but there was no difference in exposure when body weight was taken into account.
Renal Impairment
Memantine pharmacokinetics were evaluated following single oral administration of 20 mg memantine hydrochloride in 8 subjects with mild renal impairment (creatinine clearance, CLcr, > 50 – 80 mL/min), 8 subjects with moderate renal impairment (CLcr 30 – 49 mL/min), 7 subjects with severe renal impairment (CLcr 5 – 29 mL/min) and 8 healthy subjects (CLcr > 80 mL/min) matched as closely as possible by age, weight and gender to the subjects with renal impairment. Mean AUC0-∞ increased by 4%, 60%, and 115% in subjects with mild, moderate, and severe renal impairment, respectively, compared to healthy subjects. The terminal elimination half-life increased by 18%, 41%, and 95% in subjects with mild, moderate, and severe renal impairment, respectively, compared to healthy subjects.
Hepatic Impairment
Memantine pharmacokinetics were evaluated following the administration of single oral doses of 20 mg in 8 subjects with moderate hepatic impairment (Child-Pugh Class B, score 7-9) and 8 subjects who were age-, gender-, and weight-matched to the hepatically-impaired subjects. There was no change in memantine exposure (based on Cmax and AUC) in subjects with moderate hepatic impairment as compared with healthy subjects. However, terminal elimination half-life increased by about 16% in subjects with moderate hepatic impairment as compared with healthy subjects.
Drug-Drug Interactions
Use with Cholinesterase Inhibitors
Coadministration of memantine with the AChE inhibitor donepezil did not affect the pharmacokinetics of either compound. Furthermore, memantine did not affect AChE inhibition by donepezil. In a 24-week controlled clinical study in patients with moderate to severe Alzheimer’s disease, the adverse reaction profile observed with a combination of memantine immediate-release and donepezil was similar to that of donepezil alone.
Effect of Memantine on the Metabolism of Other Drugs
In vitro studies conducted with marker substrates of CYP450 enzymes (CYP1A2, -2A6, -2C9, -2D6, -2E1, -3A4) showed minimal inhibition of these enzymes by memantine. In addition, in vitro studies indicate that at concentrations exceeding those associated with efficacy, memantine does not induce the cytochrome P450 isozymes CYP1A2, -2C9, -2E1 and -3A4/5. No pharmacokinetic interactions with drugs metabolized by these enzymes are expected.
Pharmacokinetic studies evaluated the potential of memantine for interaction with warfarin and bupropion. Memantine did not affect the pharmacokinetics of the CYP2B6 substrate bupropion or its metabolite hydroxybupropion. Furthermore, memantine did not affect the pharmacokinetics or pharmacodynamics of warfarin as assessed by the prothrombin INR.
Effect of Other Drugs on Memantine
Memantine is predominantly renally eliminated, and drugs that are substrates and/or inhibitors of the CYP450 system are not expected to alter the metabolism of memantine.
Drugs Eliminated via Renal Mechanisms
Because memantine is eliminated in part by tubular secretion, coadministration of drugs that use the same renal cationic system, including hydrochlorothiazide (HCTZ), triamterene (TA), metformin, cimetidine, ranitidine, quinidine, and nicotine, could potentially result in altered plasma levels of both agents. However, coadministration of memantine and HCTZ/TA did not affect the bioavailability of either memantine or TA, and the bioavailability of HCTZ decreased by 20%. In addition, coadministration of memantine with the antihyperglycemic drug Glucovance® (glyburide and metformin hydrochloride) did not affect the pharmacokinetics of memantine, metformin and glyburide. Furthermore, memantine did not modify the serum glucose lowering effect of Glucovance®, indicating the absence of a pharmacodynamic interaction.
Drugs Highly Bound to Plasma Proteins
Because the plasma protein binding of memantine is low (45%), an interaction with drugs that are highly bound to plasma proteins, such as warfarin and digoxin, is unlikely.
Patient Information
NAMENDA XR® [Nuh-MEN-dah Eks-Are]
(memantine hydrochloride) Extended-Release Capsules
Read this Patient Information that comes with NAMENDA XR® before you start taking it and each time you get a refill. There may be new information. This information does not take the place of talking to your doctor about your medical condition or your treatment.
What is NAMENDA XR?
NAMENDA XR is a prescription medicine used for the treatment of moderate to severe dementia in people with Alzheimer’s disease. NAMENDA XR belongs to a class of medicines called N-methyl-D-aspartate (NMDA) inhibitors.
It is not known if NAMENDA XR is safe and effective in children.
Who should not take NAMENDA XR?
Do not take NAMENDA XR if you are allergic to memantine or any of the other ingredients in NAMENDA XR. See the end of this leaflet for a complete list of ingredients in NAMENDA XR.
What should I tell my doctor before taking NAMENDA XR?
Before you take NAMENDA XR, tell your doctor if you:
- have or have had seizures
- have or have had problems passing urine
- have or have had bladder or kidney problems
- have liver problems
- have any other medical conditions
- are pregnant or plan to become pregnant. It is not known if NAMENDA XR will harm your unborn baby.
- are breastfeeding or plan to breastfeed. It is not known if memantine passes into your breast milk. Talk to your doctor about the best way to feed your baby if you take NAMENDA XR.
Tell your doctor about all the medicines you take, including prescription and non-prescription medicines, vitamins, and herbal supplements.
Taking NAMENDA XR with certain other medicines may affect each other. Taking NAMENDA XR with other medicines can cause serious side effects.
Especially tell your doctor if you take:
- other NMDA antagonists such as amantadine, ketamine, and dextromethorphan
- medicines that make your urine alkaline such as carbonic anhydrase inhibitors and sodium bicarbonate
Ask your doctor or pharmacist for a list of these medicines, if you are not sure.
Know the medicines you take. Keep a list of them to show your doctor and pharmacist when you get a new medicine.
How should I take NAMENDA XR?
- Your doctor will tell you how much NAMENDA XR to take and when to take it.
- Your doctor may change your dose if needed.
- NAMENDA XR may be taken with food or without food.
- NAMENDA XR capsules may be opened and sprinkled on applesauce before swallowing, but the contents of the entire capsule should be taken and the dose should not be divided. Except when opened and sprinkled on applesauce, NAMENDA XR capsules must be swallowed whole and never crushed, divided or chewed.
- Do not use any capsules of NAMENDA XR that are damaged or show signs of tampering.
- If you are currently taking another formulation of memantine, talk to your healthcare professional about how to switch to NAMENDA XR.
- If you forget to take one dose of NAMENDA XR, do not double up on the next dose. You should take only the next dose as scheduled.
- If you have forgotten to take NAMENDA XR for several days, you should not take the next dose until you talk to your doctor.
- If you take too much NAMENDA XR, call your doctor or poison control center at 1-800-222-1222, or go to the nearest hospital emergency room right away.
What are the possible side effects of NAMENDA XR?
NAMENDA XR may cause side effects, including:
The most common side effects of NAMENDA XR include:
- headache
- diarrhea
- dizziness
These are not all the possible side effects of NAMENDA XR. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store NAMENDA XR?
Store NAMENDA XR at room temperature between 68°F to 77°F (20°C to 25°C).
What are the ingredients in NAMENDA XR?
Active ingredient: memantine hydrochloride
Inactive ingredients: sugar spheres, polyvinylpyrrolidone, hypromellose, talc, polyethylene glycol, ethylcellulose, ammonium hydroxide, oleic acid, and medium chain triglycerides
Keep NAMENDA XR and all medicines out of the reach of children.
General information about the safe and effective use of NAMENDA XR:
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not take NAMENDA XR for a condition for which it was not prescribed. Do not give NAMENDA XR to other people, even if they have the same condition. It may harm them.
This Patient Information leaflet summarizes the most important information about NAMENDA XR. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about NAMENDA XR that was written for healthcare professionals.
For more information about NAMENDA XR, go to www.namendaxr.com, or call Allergan at 1-800-678-1605.
This Patient Information has been approved by the U.S. Food and Drug Administration.
Made in Ireland
Distributed by:
Allergan USA, Inc.
Madison, NJ 07940
Licensed from Merz Pharmaceuticals GmbH & Co. KGaA
NAMENDA XR® is a registered trademark of Merz Pharma GmbH & Co. KGaA
Allergan® and its design are trademarks of Allergan, Inc.
Revised: 11/2019
© 2019 Allergan. All rights reserved.
| NAMENDA
XR
memantine hydrochloride capsule, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| NAMENDA
XR
memantine hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NAMENDA
XR
memantine hydrochloride capsule, extended release |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| NAMENDA
XR
memantine hydrochloride capsule, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NAMENDA
XR
memantine hydrochloride kit |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |