Drug Detail:Nesina (Alogliptin [ al-oh-glip-tin ])
Drug Class: Dipeptidyl peptidase 4 inhibitors
Highlights of Prescribing Information
NESINA (alogliptin) tablets, for oral use
Initial U.S. Approval: 2013
Indications and Usage for Nesina
NESINA is a dipeptidyl peptidase-4 (DPP-4) inhibitor indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1)
Limitations of Use: Should not be used in patients with type 1 diabetes mellitus. (1)
Nesina Dosage and Administration
- The recommended dosage in patients with normal renal function or mild renal impairment is 25 mg orally once daily. (2.1)
- Can be taken with or without food. (2.1)
- Adjust dosage if moderate or severe renal impairment or end-stage renal disease (ESRD). (2.2)
| Degree of Renal Impairment | Creatinine Clearance (mL/min) | Recommended Dosage |
|---|---|---|
| Moderate | ≥30 to <60 | 12.5 mg once daily |
| Severe/ESRD | <30 | 6.25 mg once daily |
Dosage Forms and Strengths
Tablets: 25 mg, 12.5 mg and 6.25 mg (3)
Contraindications
History of serious hypersensitivity to alogliptin or any of the excipients in NESINA. (4)
Warnings and Precautions
- Pancreatitis: There have been postmarketing reports of acute pancreatitis. If pancreatitis is suspected, promptly discontinue NESINA. (5.1)
- Heart failure: Consider the risks and benefits of NESINA prior to initiating treatment in patients at risk for heart failure. If heart failure develops, evaluate and manage according to current standards of care and consider discontinuation of NESINA. (5.2)
- Hypersensitivity: There have been postmarketing reports of serious hypersensitivity reactions in patients treated with NESINA such as anaphylaxis, angioedema and severe cutaneous adverse reactions, including Stevens-Johnson syndrome. If hypersensitivity reactions occur, discontinue NESINA, treat promptly, and monitor until signs and symptoms resolve. (5.3)
- Hepatic effects: Postmarketing reports of hepatic failure, sometimes fatal. Causality cannot be excluded. If liver injury is detected, promptly interrupt NESINA and assess patient for probable cause, then treat cause if possible, to resolution or stabilization. Do not restart NESINA if liver injury is confirmed and no alternative etiology can be found. (5.4)
- Hypoglycemia: Consider lowering the dosage of insulin secretagogue or insulin to reduce the risk of hypoglycemia when initiating NESINA. (5.5)
- Arthralgia: Severe and disabling arthralgia has been reported in patients taking DPP-4 inhibitors. Consider as a possible cause for severe joint pain and discontinue drug if appropriate. (5.6)
- Bullous pemphigoid: There have been postmarketing reports of bullous pemphigoid requiring hospitalization in patients taking DPP-4 inhibitors. Tell patients to report development of blisters or erosions. If bullous pemphigoid is suspected, discontinue NESINA. (5.7)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥4%) are nasopharyngitis, headache and upper respiratory tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals America, Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2023
Related/similar drugs
Mounjaro, metformin, Trulicity, Lantus, Victoza, Levemir, TresibaFull Prescribing Information
1. Indications and Usage for Nesina
NESINA is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
2. Nesina Dosage and Administration
2.1 Recommended Dosage
- The recommended dosage of NESINA is 25 mg taken orally once daily. Do not split tablets.
- NESINA may be taken with or without food [see Clinical Pharmacology (12.3)].
- Instruct patients if a dose is missed, not to double their next dose.
2.2 Patients with Renal Impairment
- Assess renal function prior to initiation of NESINA and periodically thereafter [see Use in Specific Populations (8.6)].
- No dose adjustment of NESINA is necessary for patients with mild renal impairment (creatinine clearance [CrCl] ≥60 mL/min).
- The dose of NESINA is 12.5 mg once daily for patients with moderate renal impairment (CrCl ≥30 to <60 mL/min).
- The dose of NESINA is 6.25 mg once daily for patients with severe renal impairment (CrCl ≥15 to <30 mL/min) or with end-stage renal disease (ESRD) (CrCl <15 mL/min or requiring hemodialysis). NESINA may be administered without regard to the timing of dialysis. NESINA has not been studied in patients undergoing peritoneal dialysis [see Use in Specific Populations (8.6), Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
- 25 mg tablets are light red, oval, biconvex, film-coated, with "TAK ALG-25" printed on one side.
- 12.5 mg tablets are yellow, oval, biconvex, film-coated, with "TAK ALG-12.5" printed on one side.
- 6.25 mg tablets are light pink, oval, biconvex, film-coated, with "TAK ALG-6.25" printed on one side.
4. Contraindications
NESINA is contraindicated in patients with a history of serious hypersensitivity to alogliptin or any of the excipients in NESINA. Reactions such as anaphylaxis, angioedema and severe cutaneous adverse reactions have been reported [see Warnings and Precautions (5.3), Adverse Reactions (6.2)].
5. Warnings and Precautions
5.1 Pancreatitis
Acute pancreatitis has been reported in the postmarketing setting and in randomized clinical trials. In glycemic control trials in patients with type 2 diabetes mellitus, acute pancreatitis was reported in 6 (0.2%) patients treated with NESINA 25 mg and 2 (<0.1%) patients treated with active comparators or placebo. In the EXAMINE trial (a cardiovascular outcomes trial of patients with type 2 diabetes mellitus and high cardiovascular (CV) risk), acute pancreatitis was reported in 10 (0.4%) of patients treated with NESINA and in 7 (0.3%) of patients treated with placebo.
It is unknown whether patients with a history of pancreatitis are at increased risk for pancreatitis while using NESINA.
After initiation of NESINA, patients should be observed for signs and symptoms of pancreatitis. If pancreatitis is suspected, NESINA should promptly be discontinued and appropriate management should be initiated.
5.2 Heart Failure
In the EXAMINE trial which enrolled patients with type 2 diabetes mellitus and recent acute coronary syndrome, 106 (3.9%) of patients treated with NESINA and 89 (3.3%) of patients treated with placebo were hospitalized for congestive heart failure.
Consider the risks and benefits of NESINA prior to initiating treatment in patients at risk for heart failure, such as those with a prior history of heart failure and a history of renal impairment, and observe these patients for signs and symptoms of heart failure during therapy. Patients should be advised of the characteristic symptoms of heart failure and should be instructed to immediately report such symptoms. If heart failure develops, evaluate and manage according to current standards of care and consider discontinuation of NESINA.
5.3 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with NESINA [see Adverse Reactions (6.2)]. These reactions include anaphylaxis, angioedema and severe cutaneous adverse reactions, including Stevens-Johnson syndrome. If a serious hypersensitivity reaction is suspected, discontinue NESINA, assess for other potential causes for the event and institute alternative treatment for diabetes mellitus. Use caution in patients with a history of angioedema with another dipeptidyl peptidase-4 (DPP-4) inhibitor because it is unknown whether such patients will be predisposed to angioedema with NESINA.
5.4 Hepatic Effects
There have been postmarketing reports of fatal and nonfatal hepatic failure in patients taking NESINA, although some of the reports contain insufficient information necessary to establish the probable cause [see Adverse Reactions (6.2)].
In glycemic control trials in patients with type 2 diabetes mellitus, serum alanine aminotransferase (ALT) elevations greater than three times the upper limit of normal (ULN) were reported in 1.3% of patients treated with NESINA 25 mg and 1.7% of patients treated with active comparators or placebo. In the EXAMINE trial (a cardiovascular outcomes trial of patients with type 2 diabetes mellitus and high cardiovascular (CV) risk), increases in serum alanine aminotransferase three times the upper limit of the reference range occurred in 2.4% of patients treated with NESINA and in 1.8% of patients treated with placebo.
Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have clinically significant liver enzyme elevations and if abnormal liver tests persist or worsen, NESINA should be interrupted and investigation done to establish the probable cause. NESINA should not be restarted in these patients without another explanation for the liver test abnormalities.
5.5 Hypoglycemia with Concomitant Use with Insulin or Insulin Secretagogues
Insulin and insulin secretagogues, such as sulfonylureas, are known to cause hypoglycemia. Therefore, a lower dosage of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with NESINA.
5.6 Severe and Disabling Arthralgia
There have been postmarketing reports of severe and disabling arthralgia in patients taking DPP-4 inhibitors. The time to onset of symptoms following initiation of drug therapy varied from one day to years. Patients experienced relief of symptoms upon discontinuation of the medication. A subset of patients experienced a recurrence of symptoms when restarting the same drug or a different DPP-4 inhibitor. Consider DPP-4 inhibitors as a possible cause for severe joint pain and discontinue drug if appropriate.
5.7 Bullous Pemphigoid
Postmarketing cases of bullous pemphigoid requiring hospitalization have been reported with DPP-4 inhibitor use. In reported cases, patients typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Tell patients to report development of blisters or erosions while receiving NESINA. If bullous pemphigoid is suspected, NESINA should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- Pancreatitis [see Warnings and Precautions (5.1)]
- Heart Failure [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Hepatic Effects [see Warnings and Precautions (5.4)]
- Severe and Disabling Arthralgia [see Warnings and Precautions (5.6)]
- Bullous Pemphigoid [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 14,778 patients with type 2 diabetes mellitus participated in 14 randomized, double-blind, controlled clinical trials of whom 9,052 subjects were treated with NESINA, 3,469 subjects were treated with placebo and 2,257 were treated with an active comparator. The racial distribution of patients exposed to trial medication was 71% White, 17% Asian, 6% Black or African American, 2% American Indian or Alaska Native, 0% Native Hawaiian/Other Pacific Islander and 5% Multiracial or other racial groups. The ethnic distribution was 30% Hispanic or Latino and 70% was not Hispanic or Latino. The mean duration of diabetes mellitus was seven years, the mean body mass index (BMI) was 31 kg/m2 (49% of patients had a BMI ≥30 kg/m2), and the mean age was 58 years (26% of patients ≥65 years of age). The mean exposure to NESINA was 49 weeks with 3,348 subjects treated for more than one year.
In a pooled analysis of these 14 controlled clinical trials, the overall incidence of adverse reactions was 73% in patients treated with NESINA 25 mg compared to 75% with placebo and 70% with active comparator. Overall discontinuation of therapy due to adverse reactions was 6.8% with NESINA 25 mg compared to 8.4% with placebo or 6.2% with active comparator.
Adverse reactions reported in ≥4% of adult patients treated with NESINA 25 mg and more frequently than in patients who received placebo are summarized in Table 1.
| Number of Patients (%) | |||
|---|---|---|---|
| NESINA 25 mg | Placebo | Active Comparator | |
| N=6447 | N=3469 | N=2257 | |
| Nasopharyngitis | 309 (5) | 152 (4) | 113 (5) |
| Upper Respiratory Tract Infection | 287 (4) | 121 (4) | 113 (5) |
| Headache | 278 (4) | 101 (3) | 121 (5) |
Hypoglycemia
Hypoglycemic events were documented based upon a blood glucose value and/or clinical signs and symptoms of hypoglycemia.
In the monotherapy trial, the incidence of hypoglycemia was 1.5% in patients treated with NESINA compared to 1.6% with placebo. The use of NESINA as add-on therapy to glyburide or insulin did not increase the incidence of hypoglycemia compared to placebo. In a monotherapy trial comparing NESINA to a sulfonylurea in elderly patients, the incidence of hypoglycemia was 5.4% with NESINA compared to 26% with glipizide (Table 2).
|
||
| Add-On to Glyburide (26 Weeks) | NESINA 25 mg | Placebo |
| N=198 | N=99 | |
| Overall (%) | 19 (10) | 11 (11) |
| Severe (%)† | 0 | 1 (1) |
| Add-On to Insulin (± Metformin) (26 Weeks) | NESINA 25 mg | Placebo |
| N=129 | N=129 | |
| Overall (%) | 35 (27) | 31 (24) |
| Severe (%)† | 1 (1) | 2 (2) |
| Add-On to Metformin (26 Weeks) | NESINA 25 mg | Placebo |
| N=207 | N=104 | |
| Overall (%) | 0 | 3 (3) |
| Severe (%)† | 0 | 0 |
| Add-On to Pioglitazone (± Metformin or Sulfonylurea) (26 Weeks) | NESINA 25 mg | Placebo |
| N=199 | N=97 | |
| Overall (%) | 14 (7) | 5 (5) |
| Severe (%)† | 0 | 1 (1) |
| Compared to Glipizide (52 Weeks) | NESINA 25 mg | Glipizide |
| N=222 | N=219 | |
| Overall (%) | 12 (5) | 57 (26) |
| Severe (%)† | 0 | 3 (1) |
| Compared to Metformin (26 Weeks) | NESINA 25 mg | Metformin 500 mg twice daily |
| N=112 | N=109 | |
| Overall (%) | 2 (2) | 2 (2) |
| Severe (%)† | 0 | 0 |
| Add-On to Metformin Compared to Glipizide (52 Weeks) | NESINA 25 mg | Glipizide |
| N=877 | N=869 | |
| Overall (%) | 12 (1) | 207 (4) |
| Severe (%)† | 0 | 4 (1) |
In the EXAMINE trial, the incidence of investigator reported hypoglycemia was 6.7% in patients receiving NESINA and 6.5% in patients receiving placebo. Serious adverse reactions of hypoglycemia were reported in 0.8% of patients treated with NESINA and in 0.6% of patients treated with placebo.
6.2 Postmarketing Experience
The following adverse reactions have been identified during the postmarketing use of NESINA. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: acute pancreatitis, diarrhea, constipation, nausea, ileus
Hepatobiliary Disorders: fulminant hepatic failure
Immune System Disorders: hypersensitivity reactions including anaphylaxis
Investigations: hepatic enzyme elevations
Musculoskeletal and Connective Tissue Disorders: severe and disabling arthralgia, rhabdomyolysis
Renal and Urinary Disorders: tubulointerstitial nephritis
Skin and Subcutaneous Tissue Disorders: angioedema, rash, urticaria and severe cutaneous adverse reactions including Stevens-Johnson syndrome, bullous pemphigoid
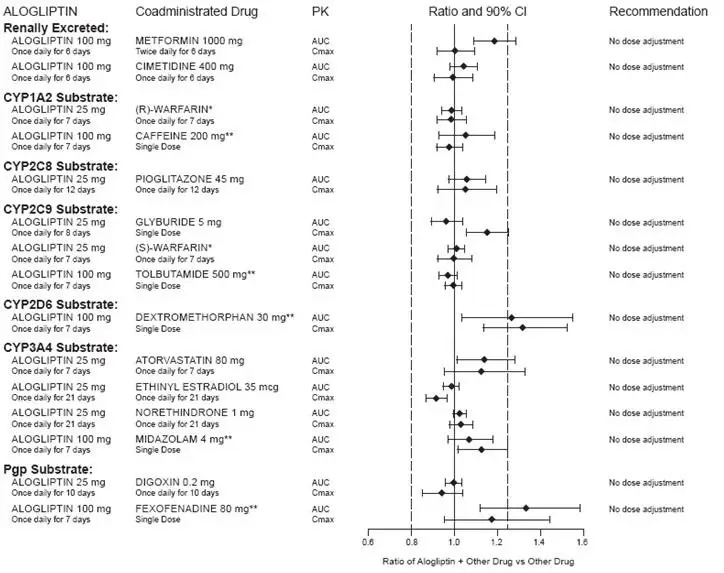
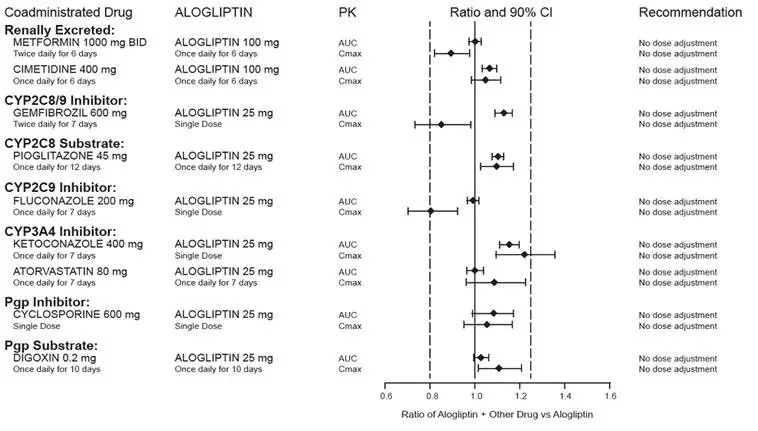
7. Drug Interactions
7.1 Insulin Secretagogues and Insulin
Insulin and insulin secretagogues are known to cause hypoglycemia. Coadministration of NESINA with an insulin secretagogue (e.g., sulfonylurea) or insulin may require lower dosages of the insulin secretagogue and insulin to reduce the risk of hypoglycemia [see Warnings and Precautions (5.5)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of NESINA have not been established in pediatric patients.
Effectiveness of NESINA was not demonstrated in a 52 week, randomized, double-blind, placebo-controlled trial (NCT02856113) in 151 pediatric patients aged 10 to 17 years with inadequately controlled type 2 diabetes mellitus.
8.5 Geriatric Use
Of the total number of patients (N=9052) in clinical safety and efficacy trials treated with NESINA, 2,257 (24.9%) patients were 65 years and older and 386 (4.3%) patients were 75 years and older. No overall differences in safety or effectiveness were observed between patients 65 years and over and younger patients.
8.6 Renal Impairment
A total of 602 adult patients with moderate renal impairment (eGFR ≥30 and <60 mL/min/1.73 m2) and 4 patients with severe renal impairment/end-stage renal disease (eGFR <30 mL/min/1.73 m2 or <15 mL/min/1.73 m2, respectively) at baseline were treated with NESINA in clinical trials in patients with type 2 diabetes mellitus.
In the EXAMINE trial of high CV risk type 2 diabetes mellitus patients, 694 patients had moderate renal impairment and 78 patients had severe renal impairment or end-stage renal disease at baseline.
The recommended dose is 12.5 mg once daily in patients with moderate renal impairment and 6.25 mg once daily in patients with severe renal impairment, as well as in patients with ESRD requiring dialysis. NESINA may be administered without regard to the timing of the dialysis.
8.7 Hepatic Impairment
No dose adjustments are required in patients with mild to moderate hepatic impairment (Child-Pugh Grade A and B) [see Clinical Pharmacology (12.3)]. NESINA has not been studied in patients with severe hepatic impairment (Child-Pugh Grade C). Use caution when administering NESINA to patients with liver disease [see Warnings and Precautions (5.4)].
10. Overdosage
In the event of an overdose, it is reasonable to institute the necessary clinical monitoring and supportive therapy as dictated by the patient's clinical status. Per clinical judgment, it may be reasonable to initiate removal of unabsorbed material from the gastrointestinal tract.
Alogliptin is minimally dialyzable; over a three-hour hemodialysis session, approximately 7% of the drug was removed. Therefore, hemodialysis is unlikely to be beneficial in an overdose situation. It is not known if NESINA is dialyzable by peritoneal dialysis.
In the event of an overdose, contact the Poison Help Line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations.
11. Nesina Description
NESINA tablets contain the active ingredient alogliptin, which is a selective, orally bioavailable inhibitor of the enzymatic activity of DPP-4.
Chemically, alogliptin is prepared as a benzoate salt, which is identified as 2-({6-[(3R)-3-aminopiperidin-1-yl]-3-methyl-2,4-dioxo-3,4-dihydropyrimidin-1(2H)-yl}methyl)benzonitrile monobenzoate. It has a molecular formula of C18H21N5O2∙C7H6O2 and a molecular weight of 461.51 daltons. The structural formula is:
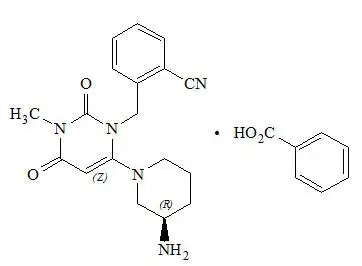
Alogliptin benzoate is a white to off-white crystalline powder containing one asymmetric carbon in the aminopiperidine moiety. It is soluble in dimethylsulfoxide, sparingly soluble in water and methanol, slightly soluble in ethanol and very slightly soluble in octanol and isopropyl acetate.
Each NESINA tablet contains 34 mg, 17 mg or 8.5 mg alogliptin benzoate, which is equivalent to 25 mg, 12.5 mg or 6.25 mg, respectively, of alogliptin and the following inactive ingredients: croscarmellose sodium, hydroxypropyl cellulose, magnesium stearate, mannitol, and microcrystalline cellulose. In addition, the film coating contains the following inactive ingredients: ferric oxide (red or yellow), hypromellose, polyethylene glycol, and titanium dioxide and is marked with printing ink (Gray F1).
12. Nesina - Clinical Pharmacology
12.1 Mechanism of Action
Increased concentrations of the incretin hormones such as glucagon-like peptide-1 (GLP-1) and glucose-dependent insulinotropic polypeptide (GIP) are released into the bloodstream from the small intestine in response to meals. These hormones cause insulin release from the pancreatic beta cells in a glucose-dependent manner but are inactivated by the DPP-4 enzyme within minutes. GLP-1 also lowers glucagon secretion from pancreatic alpha cells, reducing hepatic glucose production. In patients with type 2 diabetes mellitus, concentrations of GLP-1 are reduced but the insulin response to GLP-1 is preserved. Alogliptin is a DPP-4 inhibitor that slows the inactivation of the incretin hormones, thereby increasing their bloodstream concentrations and reducing fasting and postprandial glucose concentrations in a glucose-dependent manner in patients with type 2 diabetes mellitus. Alogliptin selectively binds to and inhibits DPP-4 but not DPP-8 or DPP-9 activity in vitro at concentrations approximating therapeutic exposures.
12.3 Pharmacokinetics
The pharmacokinetics of NESINA has been studied in healthy subjects and in patients with type 2 diabetes mellitus. The pharmacokinetics of NESINA was similar in healthy subjects and in patients with type 2 diabetes mellitus. After multiple-dose administration up to 400 mg for 14 days in patients with type 2 diabetes mellitus, accumulation of alogliptin was minimal with an increase in total [e.g., area under the plasma concentration curve (AUC)] and peak (i.e., Cmax) alogliptin exposures of 34% and 9%, respectively. Total and peak exposure to alogliptin increased proportionally across single doses and multiple doses of alogliptin ranging from 25 mg to 400 mg. The intersubject coefficient of variation for alogliptin AUC was 17%.
14. Clinical Studies
14.1 Overview of Clinical Trials in Adults with Type 2 Diabetes Mellitus
NESINA has been studied as monotherapy and in combination with metformin, a sulfonylurea, a thiazolidinedione (either alone or in combination with metformin or a sulfonylurea) and insulin (either alone or in combination with metformin).
A total of 14,053 patients with type 2 diabetes mellitus were randomized in 11 double-blind, placebo- or active-controlled clinical safety and efficacy trials conducted to evaluate the effects of NESINA on glycemic control. The racial distribution of patients exposed to trial medication was 70% White, 17% Asian, 6% Black or African American, 2% American Indian or Alaska Native, 0% Native Hawaiian/Other Pacific Islander and 5% Multiracial or other racial groups. The ethnic distribution was 30% Hispanic or Latino. Patients had an overall mean age of 57 years (range 21 to 91 years).
In patients with type 2 diabetes mellitus, treatment with NESINA produced clinically meaningful and statistically significant improvements in hemoglobin A1c (A1C) compared to placebo. As is typical for trials of agents to treat type 2 diabetes mellitus, the mean reduction in A1C with NESINA appears to be related to the degree of A1C elevation at baseline.
NESINA had similar changes from baseline in serum lipids compared to placebo.
14.2 Patients with Inadequate Glycemic Control on Diet and Exercise
A total of 1,768 patients with type 2 diabetes mellitus participated in three double-blind trials to evaluate the efficacy and safety of NESINA in patients with inadequate glycemic control on diet and exercise. All three trials had a four week, single-blind, placebo run-in period followed by a 26 week randomized treatment period. Patients who failed to meet prespecified hyperglycemic goals during the 26 week treatment periods received glycemic rescue therapy.
In a 26 week, double-blind, placebo-controlled trial, a total of 329 patients (mean baseline A1C = 8%) were randomized to receive NESINA 12.5 mg, NESINA 25 mg or placebo once daily. Treatment with NESINA 25 mg resulted in statistically significant improvements from baseline in A1C and fasting plasma glucose (FPG) compared to placebo at Week 26 (Table 3). A total of 8% of patients receiving NESINA 25 mg and 30% of those receiving placebo required glycemic rescue therapy.
Improvements in A1C were not affected by gender, age or baseline body mass index (BMI).
The mean change in body weight with NESINA was similar to placebo.
| NESINA 25 mg | Placebo | |
|---|---|---|
|
||
| A1C (%) | N=128 | N=63 |
| Baseline (mean) | 7.9 | 8.0 |
| Change from baseline (adjusted mean†) | -0.6 | 0 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -0.6‡ (-0.8, -0.3) | ˗ |
| % of patients (n/N) achieving A1C ≤7% | 44% (58/131)‡ | 23% (15/64) |
| FPG (mg/dL) | N=129 | N=64 |
| Baseline (mean) | 172 | 173 |
| Change from baseline (adjusted mean†) | -16 | 11 |
| Difference from placebo (adjusted mean† with 95% confidence interval) | -28‡ (-40, -15) | ˗ |
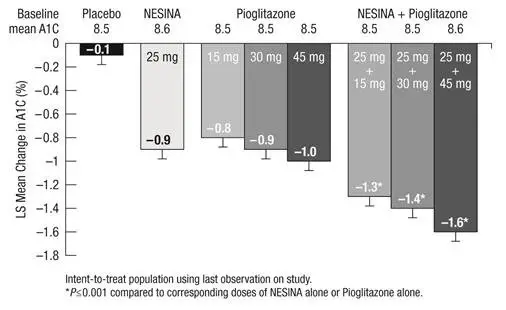
In a 26 week, double-blind, active-controlled trial, a total of 655 patients (mean baseline A1C = 8.8%) were randomized to receive NESINA 25 mg alone, pioglitazone 30 mg alone, NESINA 12.5 mg with pioglitazone 30 mg or NESINA 25 mg with pioglitazone 30 mg once daily. Coadministration of NESINA 25 mg with pioglitazone 30 mg resulted in statistically significant improvements from baseline in A1C and FPG compared to NESINA 25 mg alone and to pioglitazone 30 mg alone (Table 4). A total of 3% of patients receiving NESINA 25 mg coadministered with pioglitazone 30 mg, 11% of those receiving NESINA 25 mg alone and 6% of those receiving pioglitazone 30 mg alone required glycemic rescue.
Improvements in A1C were not affected by gender, age or baseline BMI.
The mean increase in body weight was similar between pioglitazone alone and NESINA when coadministered with pioglitazone.
| NESINA 25 mg | Pioglitazone 30 mg | NESINA 25 mg + Pioglitazone 30 mg | |
|---|---|---|---|
|
|||
| A1C (%) | N=160 | N=153 | N=158 |
| Baseline (mean) | 8.8 | 8.8 | 8.8 |
| Change from baseline (adjusted mean†) | -1.0 | -1.2 | -1.7 |
| Difference from NESINA 25 mg (adjusted mean† with 95% confidence interval) | ˗ | ˗ | -0.8‡ (-1.0, -0.5) |
| Difference from pioglitazone 30 mg (adjusted mean† with 95% confidence interval) | ˗ | ˗ | -0.6‡ (-0.8, -0.3) |
| % of patients (n/N) achieving A1C ≤7% | 24% (40/164) | 34% (55/163) | 63% (103/164)‡ |
| FPG (mg/dL) | N=162 | N=157 | N=162 |
| Baseline (mean) | 189 | 189 | 185 |
| Change from baseline (adjusted mean†) | -26 | -37 | -50 |
| Difference from NESINA 25 mg (adjusted mean† with 95% confidence interval) | ˗ | ˗ | -24‡ (-34, -15) |
| Difference from pioglitazone 30 mg (adjusted mean† with 95% confidence interval) | ˗ | ˗ | -13‡ (-22, -4) |
In a 26 week, double-blind, placebo-controlled trial, a total of 784 patients inadequately controlled on diet and exercise alone (mean baseline A1C = 8.4%) were randomized to one of seven treatment groups: placebo; metformin HCl 500 mg or metformin HCl 1000 mg twice daily; NESINA 12.5 mg twice daily; NESINA 25 mg daily; or NESINA 12.5 mg in combination with metformin HCl 500 mg or metformin HCl 1000 mg twice daily. Both coadministration treatment arms (NESINA 12.5 mg + metformin HCl 500 mg and NESINA 12.5 mg + metformin HCl 1000 mg) resulted in statistically significant improvements in A1C and FPG when compared with their respective individual alogliptin and metformin component regimens (Table 5). Coadministration treatment arms demonstrated improvements in two hour postprandial glucose (PPG) compared to NESINA alone or metformin alone (Table 5). A total of 12.3% of patients receiving NESINA 12.5 mg + metformin HCl 500 mg, 2.6% of patients receiving NESINA 12.5 mg + metformin HCl 1000 mg, 17.3% of patients receiving NESINA 12.5 mg, 22.9% of patients receiving metformin HCl 500 mg, 10.8% of patients receiving metformin HCl 1000 mg and 38.7% of patients receiving placebo required glycemic rescue.
Improvements in A1C were not affected by gender, age, race or baseline BMI. The mean decrease in body weight was similar between metformin alone and NESINA when coadministered with metformin.
| Placebo | NESINA 12.5 mg Twice Daily | Metformin HCl 500 mg Twice Daily | Metformin HCl 1000 mg Twice Daily | NESINA 12.5 mg + Metformin HCl 500 mg Twice Daily | NESINA 12.5 mg + Metformin HCl 1000 mg Twice Daily |
|
|---|---|---|---|---|---|---|
|
||||||
| A1C (%)* | N=102 | N=104 | N=103 | N=108 | N=102 | N=111 |
| Baseline (mean) | 8.5 | 8.4 | 8.5 | 8.4 | 8.5 | 8.4 |
| Change from baseline (adjusted mean†) | 0.1 | -0.6 | -0.7 | -1.1 | -1.2 | -1.6 |
| Difference from metformin (adjusted mean† with 95% confidence interval) | - | - | - | - | -0.6‡
(-0.9, -0.3) | -0.4‡
(-0.7, -0.2) |
| Difference from NESINA (adjusted mean† with 95% confidence interval) | - | - | - | - | -0.7‡
(-1.0, -0.4) | -1.0‡
(-1.3, -0.7) |
| % of patients (n/N) achieving A1C <7%§ | 4% (4/102) | 20% (21/104) | 27% (28/103) | 34% (37/108) | 47%‡
(48/102) | 59%‡
(66/111) |
| FPG (mg/dL)* | N=105 | N=106 | N=106 | N=110 | N=106 | N=112 |
| Baseline (mean) | 187 | 177 | 180 | 181 | 176 | 185 |
| Change from baseline (adjusted mean†) | 12 | -10 | -12 | -32 | -32 | -46 |
| Difference from metformin (adjusted mean† with 95% confidence interval) | - | - | - | - | -20‡
(-33, -8) | -14‡
(-26, -2) |
| Difference from NESINA (adjusted mean† with 95% confidence interval) | - | - | - | - | -22‡
(-35, -10) | -36‡
(-49, -24) |
| 2-Hour PPG (mg/dL)¶ | N=26 | N=34 | N=28 | N=37 | N=31 | N=37 |
| Baseline (mean) | 263 | 272 | 247 | 266 | 261 | 268 |
| Change from baseline (adjusted mean†) | -21 | -43 | -49 | -54 | -68 | -86‡ |
| Difference from metformin (adjusted mean† with 95% confidence interval) | - | - | - | - | -19 (-49, 11) | -32‡
(-58, -5) |
| Difference from NESINA (adjusted mean† with 95% confidence interval) | - | - | - | - | -25 (-53, -3) | -43‡
(-70, -16) |
14.4 Cardiovascular Safety Trial
A randomized, double-blind, placebo-controlled cardiovascular outcomes trial (EXAMINE) was conducted to evaluate the cardiovascular risk of NESINA. The trial compared the risk of major adverse cardiovascular events (MACE) between NESINA (N=2701) and placebo (N=2679) when added to standard of care therapies for diabetes mellitus and atherosclerotic vascular disease (ASCVD). The trial was event driven and patients were followed until a sufficient number of primary outcome events accrued.
Eligible patients were adults with type 2 diabetes mellitus who had inadequate glycemic control at baseline (e.g., HbA1c >6.5%) and had been hospitalized for an acute coronary syndrome event (e.g., acute myocardial infarction or unstable angina requiring hospitalization) 15 to 90 days prior to randomization. The dose of NESINA was based on estimated renal function at baseline per dosage and administration recommendations [see Dosage and Administration (2.2)]. The average time between an acute coronary syndrome event and randomization was approximately 48 days.
The mean age of the population was 61 years. Most patients were male (68%), White (73%), and were recruited from outside of the United States (86%). Asian and Black or African American patients contributed 20% and 4% of the total population, respectively. At the time of randomization patients had a diagnosis of type 2 diabetes mellitus for approximately 9 years, 87% had a prior myocardial infarction and 14% were current smokers. Hypertension (83%) and renal impairment (27% with an eGFR ≤60 mL/min/1.73 m2) were prevalent co-morbid conditions. Use of medications to treat diabetes mellitus (e.g., metformin 73%, sulfonylurea 54%, insulin 41%), and ASCVD (e.g., statin 94%, aspirin 93%, renin-angiotensin system blocker 88%, beta-blocker 87%) was similar between patients randomized to NESINA and placebo at baseline. During the trial, medications to treat diabetes mellitus and ASCVD could be adjusted to ensure care for these conditions adhered to standard of care recommendations set by local practice guidelines.
The primary endpoint in EXAMINE was the time to first occurrence of a MACE defined as the composite of cardiovascular death, nonfatal myocardial infarction (MI), or nonfatal stroke. The trial was designed to exclude a pre-specified risk margin of 1.3 for the hazard ratio of MACE. The median exposure to trial drug was 526 days and 95% of the patients were followed to trial completion or death.
Table 12 shows the trial results for the primary MACE composite endpoint and the contribution of each component to the primary MACE endpoint. The upper bound of the confidence interval was 1.16 and excluded a risk margin larger than 1.3.
|
|||||
| Composite of first event of CV death, nonfatal MI or nonfatal stroke (MACE) | NESINA | Placebo | Hazard Ratio | ||
| Number of Patients (%) | Rate per 100 PY* | Number of Patients (%) | Rate per 100 PY* | (98% CI) | |
| N=2701 | N=2679 | ||||
| 305 (11.3) | 7.6 | 316 (11.8) | 7.9 | 0.96 (0.80, 1.16) | |
| CV Death | 89 (3.3) | 2.2 | 111 (4.1) | 2.8 | |
| Non-fatal MI | 187 (6.9) | 4.6 | 173 (6.5) | 4.3 | |
| Non-fatal stroke | 29 (1.1) | 0.7 | 32 (1.2) | 0.8 | |
The Kaplan-Meier based cumulative event probability is presented in Figure 4 for the time to first occurrence of the primary MACE composite endpoint by treatment arm. The curves for placebo and NESINA overlap throughout the duration of the trial. The observed incidence of MACE was highest within the first 60 days after randomization in both treatment arms (14.8 MACE per 100 PY), decreased from day 60 to the end of the first year (8.4 per 100 PY) and was lowest after one year of follow-up (5.2 per 100 PY).
| Figure 4. Observed Cumulative Rate of MACE in EXAMINE |
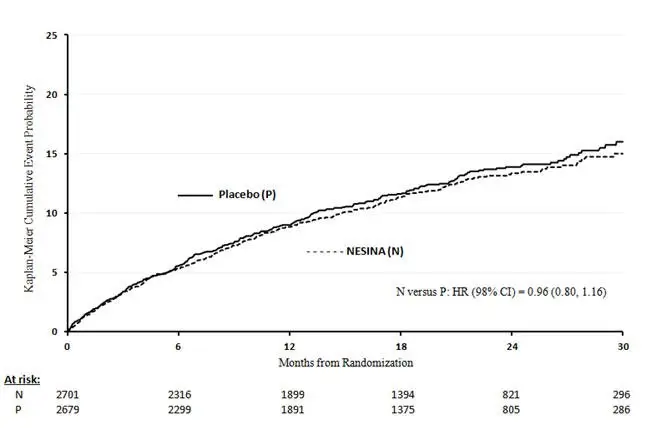 |
The rate of all cause death was similar between treatment arms with 153 (3.6 per 100 PY) recorded among patients randomized to NESINA and 173 (4.1 per 100 PY) among patients randomized to placebo. A total of 112 deaths (2.9 per 100 PY) among patients on NESINA and 130 among patients on placebo (3.5 per 100 PY) were adjudicated as cardiovascular deaths.
16. How is Nesina supplied
NESINA tablets are available as film-coated tablets containing 25 mg, 12.5 mg or 6.25 mg of alogliptin as follows:
25 mg tablet: light red, oval, biconvex, film-coated, with "TAK ALG-25" printed on one side, available in:
| NDC 64764-250-30 | Bottles of 30 tablets |
| NDC 64764-250-90 | Bottles of 90 tablets |
| NDC 64764-250-50 | Bottles of 500 tablets |
12.5 mg tablet: yellow, oval, biconvex, film-coated, with "TAK ALG-12.5" printed on one side, available in:
| NDC 64764-125-30 | Bottles of 30 tablets |
| NDC 64764-125-90 | Bottles of 90 tablets |
| NDC 64764-125-50 | Bottles of 500 tablets |
6.25 mg tablet: light pink, oval, biconvex, film-coated, with "TAK ALG-6.25" printed on one side, available in:
| NDC 64764-625-30 | Bottles of 30 tablets |
| NDC 64764-625-90 | Bottles of 90 tablets |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| NESINA
alogliptin tablet, film coated |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NESINA
alogliptin tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| NESINA
alogliptin tablet, film coated |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |