Drug Detail:Nexviazyme (Avalglucosidase alfa [ ay-val-gloo-koe-si-dase-al-fa ])
Drug Class: Lysosomal enzymes
Highlights of Prescribing Information
NEXVIAZYME (avalglucosidase alfa-ngpt) for injection, for intravenous use
Initial U.S. Approval: 2021
WARNING: SEVERE HYPERSENSITIVITY REACTIONS, INFUSION-ASSOCIATED REACTIONS, and RISK OF ACUTE CARDIORESPIRATORY FAILURE IN SUSCEPTIBLE PATIENTS
See full prescribing information for complete boxed warning.
Hypersensitivity Reactions Including Anaphylaxis
- Appropriate medical support measures, including cardiopulmonary resuscitation equipment, should be readily available. If a severe hypersensitivity reaction occurs, NEXVIAZYME should be discontinued immediately and appropriate medical treatment should be initiated. (5.1)
Infusion-Associated Reactions (IARs)
- If severe IARs occur, consider immediate discontinuation and initiation of appropriate medical treatment. (5.2)
Risk of Acute Cardiorespiratory Failure in Susceptible Patients
- Patients susceptible to fluid volume overload, or those with acute underlying respiratory illness or compromised cardiac or respiratory function, may be at risk of serious exacerbation of their cardiac or respiratory status during NEXVIAZYME infusion. (5.3)
Indications and Usage for Nexviazyme
NEXVIAZYME is a hydrolytic lysosomal glycogen-specific enzyme indicated for the treatment of patients 1 year of age and older with late-onset Pompe disease (lysosomal acid alpha-glucosidase [GAA] deficiency). (1)
Nexviazyme Dosage and Administration
- Consider administering antihistamines, antipyretics, and/or corticosteroids prior to NEXVIAZYME administration to reduce the risk of IARs. (2.1)
- Must be reconstituted and diluted prior to use.
- See full prescribing information for administration instructions including the recommended infusion rate schedule. (2.1, 2.3, 2.4)
- NEXVIAZYME is administered as intravenous infusion. For patients weighing (2.1):
- ≥30 kg, the recommended dosage is 20 mg/kg (of actual body weight) every two weeks.
- <30 kg, the recommended dosage is 40 mg/kg (of actual body weight) every two weeks.
- See the full prescribing information for dosage modifications due to hypersensitivity reactions or IARs. (2.2)
Dosage Forms and Strengths
For injection: 100 mg of avalglucosidase alfa-ngpt as a lyophilized powder in a single-dose vial for reconstitution. (3)
Contraindications
None. (4)
Warnings and Precautions
See boxed warning. (5.1, 5.2, 5.3)
Adverse Reactions/Side Effects
The most common adverse reactions (>5%) were headache, fatigue, diarrhea, nausea, arthralgia, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia and urticaria. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Genzyme at 1-800-745-4447 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 4/2023
Full Prescribing Information
WARNING: SEVERE HYPERSENSITIVITY REACTIONS, INFUSION-ASSOCIATED REACTIONS, and RISK OF ACUTE CARDIORESPIRATORY FAILURE IN SUSCEPTIBLE PATIENTS
1. Indications and Usage for Nexviazyme
NEXVIAZYME is indicated for the treatment of patients 1 year of age and older with late-onset Pompe disease (lysosomal acid alpha-glucosidase [GAA] deficiency).
2. Nexviazyme Dosage and Administration
2.1 Recommended Dosage and Administration
- Prior to NEXVIAZYME administration, consider pretreating with antihistamines, antipyretics, and/or corticosteroids [see Warnings and Precautions (5.1)].
- NEXVIAZYME must be reconstituted and diluted prior to use [see Dosage and Administration (2.3)].
- NEXVIAZYME is administered as intravenous infusion. For patients weighing:
- ≥30 kg, the recommended dosage is 20 mg/kg (of actual body weight) every two weeks [see Dosage and Administration (2.4)]
- <30 kg, the recommended dosage is 40 mg/kg (of actual body weight) every two weeks [see Dosage and Administration (2.4)]
- The initial recommended infusion rate is 1 mg/kg/hour. Gradually increase the infusion rate every 30 minutes if there are no signs of infusion-associated reactions (IARs) [see Dosage and Administration (2.4)].
2.2 Dosage and Administration Modifications Due to Hypersensitivity Reactions and/or Infusion-Associated Reactions
- In the event of a severe hypersensitivity reaction (including anaphylaxis) or a severe infusion-associated reaction (IAR), immediately discontinue NEXVIAZYME administration and initiate appropriate medical treatment [see Warnings and Precautions (5.1)].
- In the event of a mild to moderate hypersensitivity reaction or a moderate IAR, consider temporarily holding or slowing the infusion rate and initiating appropriate medical treatment [see Warnings and Precautions (5.1, 5.2)]. If symptoms:
- Persist despite temporarily holding the infusion, wait at least 30 minutes for symptoms to resolve before deciding to stop the infusion for the day.
- Subside, resume the infusion for 30 minutes at half the rate at which the reaction occurred, and subsequently increase the infusion rate by 50% for 15 minutes to 30 minutes. If symptoms do not recur, increase the infusion rate to the rate at which the reaction occurred and consider continuing to increase the rate in a stepwise manner.
2.3 Reconstitution and Dilution Instructions
Reconstitute and dilute NEXVIAZYME in the following manner. Use aseptic technique during preparation.
Storage of the Diluted Solution
- If the diluted solution is not used immediately, refrigerate at 36°F to 46°F (2°C to 8°C) for up to 24 hours. Do not freeze.
- Completely infuse the diluted solution within 9 hours after removal from the refrigerator.
- If the diluted solution is removed from the refrigerator, it must not be restored in the refrigerator.
- Discard the diluted solution if refrigerated more than 24 hours or if the diluted solution is not able to be completely infused within 9 hours after removal from the refrigerator.
| Patient Weight Range (kg) | Total Infusion Volume (mL) for 20 mg/kg | Total Infusion Volume (mL) for 40 mg/kg |
|---|---|---|
| 5 to 9.9 | N/A | 100 |
| 10 to 19.9 | N/A | 200 |
| 20 to 29.9 | N/A | 300 |
| 30 to 34.9 | 200 | N/A |
| 35 to 49.9 | 250 | N/A |
| 50 to 59.9 | 300 | N/A |
| 60 to 99.9 | 500 | N/A |
| 100 to 119.9 | 600 | N/A |
| 120 to 140 | 700 | N/A |
2.4 Administration Instructions
- It is recommended to use an in-line, low protein binding, 0.2 micrometer filter to administer NEXVIAZYME.
- Administer the infusion incrementally, as determined by the patient's response and comfort.
When the recommended dose is 20 mg/kg- Initial and Subsequent Infusions: The recommended starting infusion rate is 1 mg/kg/hour. If there are no signs of infusion-associated reactions (IARs), gradually increase the infusion rate every 30 minutes in each of the following three steps: 3 mg/kg/hour, 5 mg/kg/hour, and then 7 mg/kg/hour; then, maintain the infusion rate at 7 mg/kg/hour until the infusion is complete. The approximate total infusion duration is 4 hours to 5 hours.
When the recommended dose is 40 mg/kg- Initial Infusion: The recommended starting infusion rate is 1 mg/kg/hour. If there are no signs of IARs, gradually increase the infusion rate every 30 minutes in each of the following three steps: 3 mg/kg/hour, 5 mg/kg/hour, and then 7 mg/kg/hour; then, maintain the infusion rate at 7 mg/kg/hour until the infusion is complete (4-step process). The approximate total infusion duration is 7 hours.
- Subsequent Infusions: The recommended starting infusion rate is 1 mg/kg/hour, with gradual increase in infusion rate every 30 minutes if there are no signs of IARs. The process may use either the above 4-step process or the following 5-step process: 3 mg/kg/hour, 6 mg/kg/hour, 8 mg/kg/hour, and then 10 mg/kg/hour; then, maintain the infusion rate at 10 mg/kg/hour until the infusion is complete. The approximate total 5-step infusion duration is 5 hours.
- After the infusion is complete, flush the intravenous line with 5% Dextrose Injection.
- Discard any unused diluted product after 9 hours.
- Do not infuse NEXVIAZYME in the same intravenous line with other products.
3. Dosage Forms and Strengths
For injection: 100 mg of avalglucosidase alfa-ngpt as a white to pale-yellow lyophilized powder in a single-dose vial for reconstitution.
5. Warnings and Precautions
5.1 Hypersensitivity Reactions Including Anaphylaxis
Prior to NEXVIAZYME administration, consider pretreating with antihistamines, antipyretics, and/or corticosteroids. Appropriate medical support measures, including cardiopulmonary resuscitation equipment, should be readily available during NEXVIAZYME administration.
- If a severe hypersensitivity reaction (e.g., anaphylaxis) occurs, NEXVIAZYME should be discontinued immediately and appropriate medical treatment should be initiated. The risks and benefits of readministering NEXVIAZYME following severe hypersensitivity reaction (including anaphylaxis) should be considered. Some patients have been rechallenged using slower infusion rates at a dosage lower than the recommended dosage. In patients with severe hypersensitivity reaction, a desensitization procedure to NEXVIAZYME may be considered. If the decision is made to readminister NEXVIAZYME, ensure the patient tolerates the infusion. If the patient tolerates the infusion, the dosage (dose and/or the rate) may be increased to reach the approved recommended dosage.
- If a mild or moderate hypersensitivity reaction occurs, the infusion rate may be slowed or temporarily stopped.
Life-threatening hypersensitivity reactions, including anaphylaxis, have been reported in NEXVIAZYME-treated patients. In clinical studies, 67 (48%) NEXVIAZYME-treated patients experienced hypersensitivity reactions, including 6 (4%) patients who reported severe hypersensitivity reactions and 3 (2%) additional patients who experienced anaphylaxis; 1 (1%) patient experiencing anaphylaxis discontinued from the study. Some of the hypersensitivity reactions were IgE mediated. Anaphylaxis signs and symptoms included respiratory distress, chest discomfort, flushing, cough, erythema, lip swelling, pruritus, swollen tongue, dysphagia, and rash. Symptoms of severe hypersensitivity reactions included respiratory distress, erythema, urticaria, tongue edema, and rash. Increased incidence of hypersensitivity reactions was observed in patients with higher antidrug antibody (ADA) titers [see Adverse Reactions (6.2)].
5.2 Infusion-Associated Reactions
Antihistamines, antipyretics, and/or corticosteroids can be given prior to NEXVIAZYME administration to reduce the risk of infusion-associated reactions (IARs). However, IARs may still occur in patients after receiving pretreatment.
If severe IARs occur, consider immediate discontinuation of NEXVIAZYME, initiation of appropriate medical treatment, and the benefits and risks of readministering NEXVIAZYME following severe IARs. Some patients have been rechallenged using slower infusion rates at a dose lower than the recommended dose. Once a patient tolerates the infusion, the dose may be increased to reach the recommended approved dose.
If mild or moderate IARs occur regardless of pretreatment, decreasing the infusion rate or temporarily stopping the infusion may ameliorate the symptoms.
In clinical studies, IARs were reported to occur at any time during and/or within a few hours after the NEXVIAZYME infusion and were more likely to occur with higher infusion rates. IARs were reported in 48 (34%) NEXVIAZYME-treated patients in clinical studies. In these studies, 5 (4%) NEXVIAZYME-treated patients reported 10 severe IARs including symptoms of chest discomfort, nausea, dysphagia, erythema, respiratory distress, tongue edema, urticaria, and increased blood pressure. The majority of IARs were assessed as mild to moderate. IARs that led to treatment discontinuation were chest discomfort, cough, dizziness, erythema, flushing, nausea, ocular hyperemia, and respiratory distress. Increased incidence of IARs was observed in patients with higher ADA titers [see Adverse Reactions (6.2)].
Patients with an acute underlying illness at the time of NEXVIAZYME infusion appear to be at greater risk for IARs. Patients with advanced Pompe disease may have compromised cardiac and respiratory function, which may predispose them to a higher risk of severe complications from IARs.
5.3 Risk of Acute Cardiorespiratory Failure in Susceptible Patients
Patients susceptible to fluid volume overload, or those with acute underlying respiratory illness or compromised cardiac or respiratory function for whom fluid restriction is indicated may be at risk of serious exacerbation of their cardiac or respiratory status during the NEXVIAZYME infusion. More frequent monitoring of vitals should be performed during NEXVIAZYME infusion in these patients. Some patients may require prolonged observation times.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in other sections of the labeling:
- Hypersensitivity Reactions Including Anaphylaxis [see Warnings and Precautions (5.1)]
- Infusion-Associated Reactions (IARs) [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions from Clinical Trials in Late-Onset Pompe Disease (LOPD)
In Study 1, 100 patients aged 16 to 78 years of age with LOPD (naive to enzyme replacement therapy) were treated with either 20 mg/kg of NEXVIAZYME (n=51) or 20 mg/kg of alglucosidase alfa (n=49) given every other week as an intravenous infusion for 49 weeks followed by an open-label extension period [see Clinical Studies (14.1)].
During the double-blind active-controlled period of 49 weeks, serious adverse reactions were reported in 1 (2%) patient treated with NEXVIAZYME and in 3 (6%) patients treated with alglucosidase alfa. The most frequently reported adverse reactions in (>5%) NEXVIAZYME-treated patients were headache, fatigue, diarrhea, nausea, arthralgia, dizziness, myalgia, pruritus, vomiting, dyspnea, erythema, paresthesia, and urticaria.
IARs were reported in 13 (25%) of the NEXVIAZYME-treated patients. IARs reported in more than 1 patient on NEXVIAZYME were mild to moderate and included headache, diarrhea, pruritus, urticaria, and rash. None of them were severe IARs. IARs were reported in 16 (33%) patients treated with alglucosidase alfa. IARs reported in more than 1 patient on alglucosidase alfa were mild to severe and included dizziness, flushing, dyspnea, nausea, pruritis, rash, erythema, chills, and feeling hot. Severe IARs were reported in 2 patients treated with alglucosidase alfa.
Table 2 summarizes the adverse reactions that occurred in at least 3 NEXVIAZYME-treated patients (≥6%) in Study 1. Study 1 was not designed to demonstrate a statistically significant difference in the incidence of adverse reactions in the NEXVIAZYME and the alglucosidase alfa treatment groups.
| Adverse Reaction | NEXVIAZYME (N=51) n (%) | Alglucosidase Alfa (N=49) n (%) |
|---|---|---|
| Headache | 11 (22%) | 16 (33%) |
| Fatigue | 9 (18%) | 7 (14%) |
| Diarrhea | 6 (12%) | 8 (16%) |
| Nausea | 6 (12%) | 7 (14%) |
| Arthralgia | 5 (10%) | 8 (16%) |
| Dizziness | 5 (10%) | 4 (8%) |
| Myalgia | 5 (10%) | 7 (14%) |
| Pruritus | 4 (8%) | 4 (8%) |
| Vomiting | 4 (8%) | 3 (6%) |
| Dyspnea | 3 (6%) | 4 (8%) |
| Erythema | 3 (6%) | 3 (6%) |
| Paresthesia | 3 (6%) | 2 (4%) |
| Urticaria | 3 (6%) | 1 (2%) |
6.2 Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other avalglucosidase alfa products may be misleading.
The incidence of anti-avalglucosidase alfa-ngpt antibodies (antidrug antibodies, ADA) in NEXVIAZYME-treated patients with Pompe disease is shown in Table 3. In NEXVIAZYME-treated patients (mean of 26 months, up to 85 months of treatment), the incidence of IAR was 62% (8/13) in those with an ADA peak titer ≥12,800, compared with incidences of 19% (8/43) in those with ADA peak titer <12,800 and 33% (1/3) in those who were ADA-negative [see Warnings and Precautions (5.2)]. Increased incidence of hypersensitivity reactions was observed in patients with higher ADA titers (4/13, 31%) compared to lower ADA titers (2/14, 14%). In enzyme replacement therapy (ERT)–experienced adult patients, the occurrences of IARs and hypersensitivity reactions were higher in patients who developed ADA compared to patients who were ADA-negative. One (1) treatment-naive patient (ADA peak titer 3,200) and 2 treatment-experienced patients (ADA peak titers; 800 and 12,800, respectively) developed anaphylaxis [see Warnings and Precautions (5.1)].
The median time to seroconversion was 8 weeks. No clear trend of ADA impact on pharmacokinetics was observed [see Clinical Pharmacology (12.3)]. A trend toward decreased pharmacodynamic response as measured by percent change of urinary glucose tetrasaccharides from baseline was observed in patients with ADA peak titer ≥12,800. The development of ADA did not have an apparent impact on clinical efficacy.
ADA cross-reactivity studies showed that antibodies to avalglucosidase alfa-ngpt were cross-reactive to alglucosidase alfa.
| NEXVIAZYME | ||||
|---|---|---|---|---|
| Treatment-Naive Patients Avalglucosidase alfa-ngpt ADA*
(N=61)† | Treatment-Experienced Patients Avalglucosidase alfa-ngpt ADA (N=74)‡ |
|||
| Adults/Pediatrics 20 mg/kg every two weeks (N=61)† | Adults 20 mg/kg every two weeks (N=58) | Pediatrics 20 mg/kg every two weeks (N=6) | Pediatrics 40 mg/kg every two weeks (N=10) |
|
| n (%) | n (%) | n (%) | n (%) | |
|
||||
| ADA at baseline | 2 (3%) | 43 (74%) | 1 (17%) | 1 (10%) |
| ADA after treatment | 58 (95%) | 32 (55%) | 1 (17%) | 5 (50%) |
| Neutralizing Antibody (NAb) | ||||
| Both NAb types | 13 (21%) | 3 (5%) | 0 | 0 |
| Inhibition of enzyme activity | 17 (28%) | 10 (18%) | 0 | 0 |
| Inhibition of enzyme cellular uptake | 24 (39%) | 12 (21%) | 0 | 1 (10%) |
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of NEXVIAZYME for the treatment of late-onset Pompe disease have been established in pediatric patients 1 year of age and older. Use of NEXVIAZYME for this indication is supported by evidence from two clinical studies which included adults with LOPD, and 1 pediatric patient with LOPD (16 years of age) and from safety experience in 19 pediatric patients with infantile-onset Pompe disease (IOPD) (1 to 12 years of age) treated with NEXVIAZYME [see Clinical Studies (14.1)]. NEXVIAZYME is not approved for the treatment of IOPD.
The safety profile of NEXVIAZYME in pediatric patients 1 to 12 years old with Pompe disease was similar to the safety profile of NEXVIAZYME in older pediatric and adult patients with LOPD. The safety and effectiveness of NEXVIAZYME have not been established in pediatric patients younger than 1 year of age.
11. Nexviazyme Description
Avalglucosidase alfa-ngpt is a hydrolytic lysosomal glycogen-specific recombinant human α-glucosidase enzyme conjugated with multiple synthetic bis-mannose-6-phosphate (bis-M6P)-tetra-mannose glycans resulting in approximately 15 moles of M6P per mole of enzyme (15 M6P) and is produced in Chinese hamster ovary cells (CHO). Avalglucosidase alfa-ngpt has a molecular weight of approximately 124 kDa.
NEXVIAZYME (avalglucosidase alfa-ngpt) for injection is a sterile white to pale-yellow lyophilized powder for intravenous use after reconstitution and dilution. Each single-dose vial contains 100 mg of avalglucosidase alfa-ngpt, glycine (200 mg), L-Histidine (10.7 mg), L-Histidine HCl monohydrate (6.5 mg), mannitol (200 mg), and polysorbate 80 (1 mg). After reconstitution with 10 mL of Sterile Water for Injection, USP, the resultant concentration is 100 mg/10 mL (10 mg/mL) with a pH of approximately 6.2.
12. Nexviazyme - Clinical Pharmacology
12.1 Mechanism of Action
Pompe disease (also known as glycogen storage disease type II, acid maltase deficiency, and glycogenosis type II) is an inherited disorder of glycogen metabolism caused by a deficiency of the lysosomal enzyme acid α-glucosidase (GAA), which results in intralysosomal accumulation of glycogen in various tissues.
Avalglucosidase alfa-ngpt provides an exogenous source of GAA. The M6P on avalglucosidase alfa-ngpt mediates binding to M6P receptors on the cell surface with high affinity. After binding, it is internalized and transported into lysosomes where it undergoes proteolytic cleavage that results in increased GAA enzymatic activity. Avalglucosidase alfa-ngpt then exerts enzymatic activity in cleaving glycogen.
12.2 Pharmacodynamics
In patients with Pompe disease, excess of glycogen is degraded to hexose tetrasaccharide (Hex4) which is then excreted in urine. The urinary Hex4 assay measures its major component, glucose tetrasaccharide (Glc4). In clinical studies, treatment with NEXVIAZYME resulted in reductions of urinary Glc4 concentrations (normalized by urine creatinine and reported as mmol Glc4/mol creatinine) in patients with Pompe disease.
In Study 1, the baseline mean urinary Glc4 concentration was 12.7 mmol/mol and 8.7 mmol/mol in NEXVIAZYME and alglucosidase alfa treatment groups, respectively, in treatment-naive LOPD patients [see Clinical Studies (14.1)]. The mean percentage (SD) change in urinary Glc4 concentrations from baseline to Week 49 was -54% (24) and -11% (32) in the NEXVIAZYME and alglucosidase alfa treatment groups, respectively.
12.3 Pharmacokinetics
The avalglucosidase alfa-ngpt exposure increases in an approximately proportional manner with increasing doses over a range from 5 to 20 mg/kg (0.25 to 1 time the approved recommended dosage in LOPD patients weighing ≥30 kg or 0.125 to 0.5 times the approved recommended dosage in LOPD patients weighing <30 kg). No accumulation was observed following every two weeks dosing. Following intravenous infusion of 20 mg/kg of NEXVIAZYME every two weeks in LOPD patients weighing ≥30 kg, the mean ± SD plasma Cmax of avalglucosidase alfa-ngpt at Week 1 and Week 49 was 259 ± 72 µg/mL and 242 ± 81 µg/mL, respectively; the mean ± SD plasma AUC of avalglucosidase alfa-ngpt at Week 1 and Week 49 was 1,290 ± 420 µg∙h/mL and 1,250 ± 433 µg∙h/mL, respectively. Patients weighing <30 kg are expected to have similar AUC following intravenous infusion of 40 mg/kg of NEXVIAZYME every two weeks.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential or studies to evaluate mutagenic potential have not been performed with avalglucosidase alfa-ngpt.
Intravenous administration of avalglucosidase alfa-ngpt every other day at doses up to 50 mg/kg (exposure not evaluated) had no adverse effects on fertility in male or female mice.
14. Clinical Studies
14.1 Clinical Trial in Patients with Late-Onset Pompe Disease
Study 1 (NCT02782741) was a randomized, double-blinded, multinational, multicenter trial comparing the efficacy and safety of NEXVIAZYME to alglucosidase alfa in 100 treatment-naive patients with LOPD. Patients were randomized in a 1:1 ratio based on baseline forced vital capacity (FVC), gender, age, and country to receive 20 mg/kg of NEXVIAZYME or alglucosidase alfa administered intravenously once every two weeks for 49 weeks. The trial included an open-label, long-term, follow-up phase of up to 5 years, in which patients in the alglucosidase alfa arm were switched to NEXVIAZYME treatment. Of the 100 randomized patients, 52 were males, the baseline median age was 49 years old (range from 16 to 78), median baseline weight was 76.4 kg (range from 38 to 139 kg), median length of time since diagnosis was 6.9 months (range from 0.3 to 328.4 months), mean age at diagnosis was 46.4 years old (range from 11 to 78), mean FVC (% predicted) at baseline was 62.1% (range from 32 to 85%), and mean 6MWT at baseline was 388.9 meters (range from 118 to 630 meters).
Endpoints and Results from the 49-Week Active-Controlled Period in Study 1
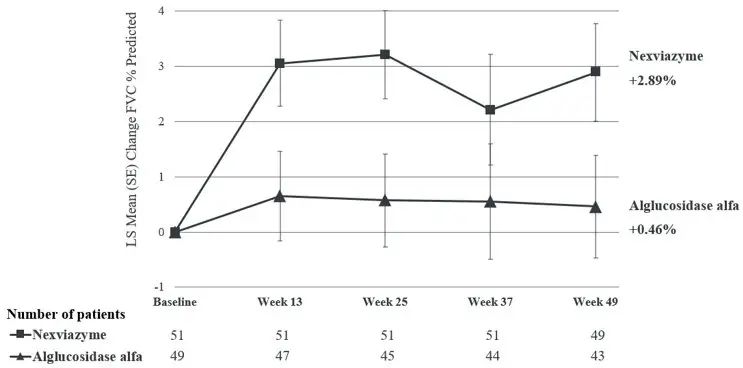
The primary endpoint of Study 1 was the change in FVC (% predicted) in the upright position from baseline to Week 49. At Week 49, the least squares (LS) mean change in FVC (% predicted) for patients treated with NEXVIAZYME and alglucosidase alfa was 2.9% and 0.5%, respectively. The estimated treatment difference was 2.4% (95% CI: -0.1, 5.0) favoring NEXVIAZYME (see Table 4). Figure 1 presents the LS mean change from baseline in FVC (% predicted) over time by treatment group up to Week 49.
| NEXVIAZYME (n=51) | Alglucosidase Alfa (n=49) |
||
|---|---|---|---|
|
|||
| Pretreatment baseline | Mean (SD) | 62.5 (14.4) | 61.6 (12.4) |
| Week 49 | Mean (SD) | 65.5 (17.4) | 61.2 (13.5) |
| Estimated change from baseline to week 49 | LS mean (SE) | 2.9† (0.9) | 0.5† (0.9) |
| Estimated difference between groups in change from baseline to week 49 | LS mean (95% CI) | 2.4†‡ (-0.1, 5.0) | |
| Figure 1: Plot of LS Mean (SE) Change from Baseline of FVC (% predicted) in Upright Position over Time in Treatment-Naive Patients with LOPD (Study 1)* |
|---|
|
|
|
The key secondary endpoint of Study 1 was change in total distance walked in 6 minutes (6-Minute Walk Test, 6MWT) from baseline to Week 49. At Week 49, the LS mean change from baseline in 6MWT for patients treated with NEXVIAZYME and alglucosidase alfa was 32.2 meters and 2.2 meters, respectively. The estimated treatment difference was 30 meters (95% CI: 1.3, 58.7) favoring NEXVIAZYME (Table 5). Figure 2 presents the LS mean change from baseline in 6MWT distance over time by treatment group.
| NEXVIAZYME (n=51) | Alglucosidase Alfa (n=49) |
||
|---|---|---|---|
|
|||
| Pretreatment baseline | Mean (SD) | 399.3 (110.9) | 378.1 (116.2) |
| Week 49 | Mean (SD) | 441.3 (109.8) | 383.6 (141.1) |
| Estimated change from baseline to week 49 | LS mean (SE) | 32.2† (9.9) | 2.2† (10.4) |
| Estimated difference between groups in change from baseline to week 49 | LS mean (95% CI) | 30.0†‡ (1.3, 58.7) | |
| Figure 2: Plot of LS Mean (SE) Change from Baseline of 6MWT (distance walked, in meters) over Time in Treatment-Naive Patients with LOPD (Study 1)* |
|---|
|
|
|
16. How is Nexviazyme supplied
NEXVIAZYME (avalglucosidase alfa-ngpt) for injection is supplied as a sterile, white to pale-yellow lyophilized powder in single-dose vials.
One 100 mg vial in a carton: NDC 58468-0426-1
| NEXVIAZYME
NGPT
avalglucosidase alfa injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Genzyme Corporation (025322157) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Flanders | 372153895 | ANALYSIS(58468-0426) , API MANUFACTURE(58468-0426) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Ireland Limited | 985127419 | ANALYSIS(58468-0426) , MANUFACTURE(58468-0426) , PACK(58468-0426) , LABEL(58468-0426) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Genzyme Corporation | 050424395 | LABEL(58468-0426) , PACK(58468-0426) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Eurofins Biopharma Product Testing Ireland Limited | 238239933 | ANALYSIS(58468-0426) | |