Drug Detail:Nicardipine (monograph) (Cardene)
Drug Class:
Nicardipine Description
Nicardipine hydrochloride is a dihydropyridine structure with the IUPAC (International Union of Pure and Applied Chemistry) chemical name 2-(benzyl-methyl amino)ethyl methyl 1,4-dihydro-2,6-dimethyl- 4-(m-nitrophenyl)-3,5-pyridinedicarboxylate monohydrochloride, and it has the following structure:
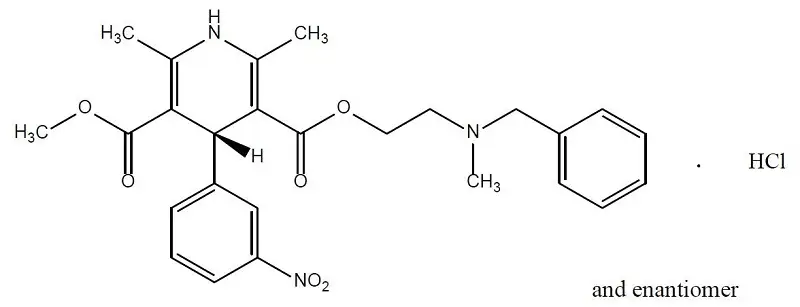
Molecular formula: C26H29N3O6 ● HCl
Nicardipine hydrochloride is a pale greenish-yellow, crystalline powder that melts at about 169°C. It is soluble in methanol, sparingly soluble in ethanol, slightly soluble in acetone, chloroform and water. It has a molecular weight of 515.99.
Each capsule, for oral administration, contains 20 mg or 30 mg of nicardipine hydrochloride. In addition, each capsule contains the following inactive ingredients: colloidal silicon dioxide, magnesium stearate and pregelatinized starch with capsule shell composed of gelatin, titanium dioxide and FD&C blue #1. Imprinting ink composed of black iron oxide, potassium hydroxide, propylene glycol, shellac and strong ammonia solution.
Nicardipine - Clinical Pharmacology
Pharmacokinetics and Metabolism
When nicardipine hydrochloride capsules were administered 1 or 3 hours after a high-fat meal, the mean Cmax and mean AUC were lower (20% to 30%) than when nicardipine hydrochloride capsules were given to fasting subjects. These decreases in plasma levels observed following a meal may be significant, but the clinical trials establishing the efficacy and safety of nicardipine hydrochloride capsules were done in patients without regard to the timing of meals. Thus, the results of these trials reflect the effects of meal-induced variability.
The pharmacokinetics of nicardipine hydrochloride capsules are nonlinear due to saturable hepatic first pass metabolism. Following oral administration, increasing doses result in a disproportionate increase in plasma levels. Steady-state Cmax values following 20-, 30-, and 40-mg doses every 8 hours averaged 36, 88, and 133 ng/mL, respectively. Hence, increasing the dose from 20 to 30 mg every 8 hours more than doubled Cmax and increasing the dose from 20 to 40 mg every 8 hours increased Cmax more than threefold. A similar disproportionate increase in AUC with dose was observed.
Considerable inter-subject variability in plasma levels was also observed.
Post-absorption kinetics of nicardipine hydrochloride capsules are also non-linear, although there is a reproducible terminal plasma half-life that averaged 8.6 hours following 30- and 40-mg doses at steady-state (tid). The terminal half-life represents the elimination of less than 5% of the absorbed drug (measured by plasma concentrations). Elimination over the first 8 hours after dosing is much faster with a half-life of 2 to 4 hours. Steady-state plasma levels are achieved after 2 to 3 days of tid dosing (every 8 hours) and are twofold higher than after a single dose.
Nicardipine hydrochloride capsules are highly protein bound (>95%) in human plasma over a wide concentration range.
Nicardipine hydrochloride capsules are metabolized extensively by the hepatic cytochrome P450 enzymes, CYP2C8, 2D6, and 3A4; less than 1% of intact drug is detected in the urine. Following a radioactive oral dose in solution, 60% of the radioactivity was recovered in the urine and 35% in feces. Most of the dose (over 90%) was recovered within 48 hours of dosing. Nicardipine hydrochloride capsules do not induce its own metabolism, however, nicardipine causes inhibition of certain cytochrome P450 enzymes (including CYP3A4, CYP2D6, CYP2C8, and CYP2C19). Inhibition of these enzymes may result in increased plasma levels of certain drugs, including cyclosporine and tacrolimus (see Drug Interactions). The altered pharmacokinetics may necessitate dosage adjustment of the affected drug or discontinuation of treatment.
Nicardipine hydrochloride plasma levels were higher in patients with mild renal impairment (baseline serum creatinine concentration ranged from 1.2 to 5.5 mg/dL) than in normal subjects. After 30-mg nicardipine hydrochloride tid at steady-state, Cmax and AUC were approximately twofold higher in these patients.
Because nicardipine hydrochloride capsules are extensively metabolized by the liver, the plasma levels of the drug are influenced by changes in hepatic function. Nicardipine hydrochloride plasma levels were higher in patients with severe liver disease (hepatic cirrhosis confirmed by liver biopsy or presence of endoscopically-confirmed esophageal varices) than in normal subjects. After 20-mg nicardipine hydrochloride capsules bid at steady-state, Cmax and AUC were 1.8 and fourfold higher, and the terminal half-life was prolonged to 19 hours in these patients.
Hemodynamics
"Coronary Steal", the detrimental redistribution of coronary blood flow in patients with coronary artery disease (diversion of blood from under perfused areas toward better perfused areas), has not been observed during nicardipine treatment. On the contrary, nicardipine has been shown to improve systolic shortening in normal and hypokinetic segments of myocardial muscle, and radio-nuclide angiography has confirmed that wall motion remained improved during an increase in oxygen demand. Nonetheless, occasional patients have developed increased angina upon receiving nicardipine. Whether this represents steal in those patients, or is the result of increased heart rate and decreased diastolic pressure, is not clear.
In patients with coronary artery disease nicardipine improves L.V. diastolic distensibility during the early filling phase, probably due to a faster rate of myocardial relaxation in previously under perfused areas. There is little or no effect on normal myocardium, suggesting the improvement is mainly by indirect mechanisms such as afterload reduction, and reduced ischemia. Nicardipine has no negative effect on myocardial relaxation at therapeutic doses. The clinical consequences of these properties are as yet undemonstrated.
Electrophysiologic Effects
Nicardipine hydrochloride increased the heart rate when given intravenously during acute electrophysiologic studies and prolonged the corrected QT interval to a minor degree. The sinus node recovery times and SA conduction times were not affected by the drug. The PA, AH, and HV intervals 1 and the functional and effective refractory periods of the atrium were not prolonged by nicardipine hydrochloride capsules and the relative and effective refractory periods of the His-Purkinje system were slightly shortened after intravenous nicardipine hydrochloride.
1PA = conduction time from high to low right atrium, AH = conduction time from low right atrium to His bundle deflection or AV nodal conduction time, HV = conduction time through the His bundle and the bundle branch-Purkinje system.
Effects in Hypertension
Table 1
| SYSTOLIC BP (mm Hg)
| DIASTOLIC BP (mm Hg)
|
||||||||
| Dose
| Number of Patients
| Mean Peak Response
| Mean Trough Response
| Trough/ Peak
| Dose
| Number of Patients
| Mean Peak Response
| Mean Trough Response
| Trough/ Peak
|
| 20 mg | 50 | -10.3 | -4.9 | 48% | 20 mg | 50 | -10.6 | -4.6 | 43% |
| 52 | -17.6 | -7.9 | 45% | 52 | -9.0 | -2.9 | 32% |
||
| 30 mg | 45 | -14.5 | -7.2 | 50% | 30 mg | 45 | -12.8 | -4.9 | 38% |
| 44 | -14.6 | -7.5 | 51% | 44 | -14.2 | -4.3 | 30% |
||
| 40 mg | 50 | -16.3 | -9.5 | 58% | 40 mg | 50 | -15.4 | -5.9 | 38% |
| 38 | -15.9 | -6.0 | 38% | 38 | -14.8 | -3.7 | 25% |
||
When added to beta-blocker therapy, nicardipine hydrochloride capsules further lower both systolic and diastolic blood pressure.
Indications and Usage for Nicardipine
Nicardipine hydrochloride capsules are indicated for the management of patients with chronic stable angina (effort-associated angina). Nicardipine hydrochloride capsules may be used alone or in combination with beta-blockers.
II. Hypertension
Nicardipine hydrochloride capsules are indicated for the treatment of hypertension. Nicardipine hydrochloride capsules may be used alone or in combination with other antihypertensive drugs. In administering nicardipine hydrochloride it is important to be aware of the relatively large peak to trough differences in blood pressure effect (See DOSAGE AND ADMINISTRATION).
Warnings
About 7% of patients in short-term, placebo-controlled angina trials have developed increased frequency, duration or severity of angina on starting nicardipine hydrochloride capsules or at the time of dosage increases, compared with 4% of patients on placebo. Comparisons with beta-blockers also show a greater frequency of increased angina, 4% vs 1%. The mechanism of this effect has not been established (see ADVERSE REACTIONS).
Use in Patients With Congestive Heart Failure
Although preliminary hemodynamic studies in patients with congestive heart failure have shown that nicardipine hydrochloride capsules reduced afterload without impairing myocardial contractility, it has a negative inotropic effect in vitro and in some patients. Caution should be exercised when using the drug in congestive heart failure patients, particularly in combination with a beta-blocker.
Beta-Blocker Withdrawal
Nicardipine hydrochloride capsules are not a beta-blocker and therefore gives no protection against the dangers of abrupt beta-blocker withdrawal; any such withdrawal should be by gradual reduction of the dose of beta-blocker, preferably over 8 to 10 days.
Precautions
Blood Pressure
Because nicardipine hydrochloride capsules decrease peripheral resistance, careful monitoring of blood pressure during the initial administration and titration of nicardipine hydrochloride capsules are suggested. Nicardipine hydrochloride capsules like other calcium channel blockers, may occasionally produce symptomatic hypotension. Caution is advised to avoid systemic hypotension when administering the drug to patients who have sustained an acute cerebral infarction or hemorrhage. Because of prominent effects at the time of peak blood levels, initial titration should be performed with measurements of blood pressure at peak effect (1 to 2 hours after dosing) and just before the next dose.
Use in Patients With Impaired Hepatic Function: Since the liver is the major site of biotransformation and since nicardipine hydrochloride capsules are subject to first pass metabolism, the drug should be used with caution in patients having impaired liver function or reduced hepatic blood flow. Patients with severe liver disease developed elevated blood levels (fourfold increase in AUC) and prolonged half- life (19 hours) of nicardipine (see DOSAGE AND ADMINISTRATION).
Use in Patients With Impaired Renal Function: When nicardipine hydrochloride capsules 20 mg or 30 mg tid was given to hypertensive patients with mild renal impairment, mean plasma concentrations, AUC and Cmax were approximately twofold higher in renally impaired patients than in healthy controls.
Doses in these patients must be adjusted (see CLINICAL PHARMACOLOGY and DOSAGE AND ADMINISTRATION).
Drug Interactions
In controlled clinical studies, adrenergic beta-receptor blockers have been frequently administered concomitantly with nicardipine hydrochloride capsules. The combination is well tolerated.
Cimetidine
Cimetidine increases nicardipine hydrochloride capsules plasma levels. Patients receiving the two drugs concomitantly should be carefully monitored.
Digoxin
Some calcium blockers may increase the concentration of digitalis preparations in the blood. Nicardipine hydrochloride capsules usually do not alter the plasma levels of digoxin; however, serum digoxin levels should be evaluated after concomitant therapy with nicardipine hydrochloride capsules are initiated.
Maalox®
Coadministration of Maalox TC had no effect on nicardipine hydrochloride capsules absorption.
Fentanyl Anesthesia
Severe hypotension has been reported during fentanyl anesthesia with concomitant use of a beta-blocker and a calcium channel blocker. Even though such interactions were not seen during clinical studies with nicardipine hydrochloride capsules, an increased volume of circulating fluids might be required if such an interaction were to occur.
Cyclosporine
Concomitant administration of oral or intravenous nicardipine and cyclosporine results in elevated plasma cyclosporine levels through nicardipine inhibition of hepatic microsomal enzymes, including CYP3A4. Plasma concentrations of cyclosporine should therefore be closely monitored, and its dosage reduced accordingly, in patients treated with nicardipine.
Tacrolimus: Concomitant administration of oral or intravenous nicardipine and tacrolimus may result in elevated plasma tacrolimus levels through nicardipine inhibition of hepatic microsomal enzymes, including CYP3A4. Closely monitor plasma concentrations of tacrolimus during nicardipine administration, and adjust the dose of tacrolimus accordingly.
When therapeutic concentrations of furosemide, propranolol, dipyridamole, warfarin, quinidine or naproxen were added to human plasma (in vitro), the plasma protein binding of nicardipine hydrochloride capsules were not altered.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Rats treated with nicardipine in the diet (at concentrations calculated to provide daily dosage levels of 5, 15 or 45 mg/kg/day) for 2 years showed a dose-dependent increase in thyroid hyperplasia and neoplasia (follicular adenoma/carcinoma). One- and 3 month studies in the rat have suggested that these results are linked to a nicardipine-induced reduction in plasma thyroxine (T4) levels with a consequent increase in plasma levels of thyroid stimulating hormone (TSH). Chronic elevation of TSH is known to cause hyperstimulation of the thyroid. In rats on an iodine deficient diet, nicardipine administration for 1 month was associated with thyroid hyperplasia that was prevented by T4 supplementation. Mice treated with nicardipine in the diet (at concentrations calculated to provide daily dosage levels of up to 100 mg/kg/day) for up to 18 months showed no evidence of neoplasia of any tissue and no evidence of thyroid changes. There was no evidence of thyroid pathology in dogs treated with up to 25 mg nicardipine/kg/day for 1 year and no evidence of effects of nicardipine on thyroid function (plasma T4 and TSH) in man.
There was no evidence of a mutagenic potential of nicardipine in a battery of genotoxicity tests conducted on microbial indicator organisms, in micronucleus tests in mice and hamsters, or in a sister chromatid exchange study in hamsters.
No impairment of fertility was seen in male or female rats administered nicardipine at oral doses as high as 100 mg/kg/day (50 times the 40 mg tid maximum recommended antianginal or antihypertensive dose in man, assuming a patient weight of 60 kg).
Pregnancy
Nicardipine was embryocidal when administered orally to pregnant Japanese White rabbits, during organogenesis, at 150 mg/kg/day (a dose associated with marked body weight gain suppression in the treated doe) but not at 50 mg/kg/day (25 times the maximum recommended antianginal or antihypertensive dose in man). No adverse effects on the fetus were observed when New Zealand albino rabbits were treated, during organogenesis, with up to 100 mg nicardipine/kg/day (a dose associated with significant mortality in the treated doe). In pregnant rats administered nicardipine orally at up to 100 mg/kg/day (50 times the maximum recommended human dose) there was no evidence of embryolethality or teratogenicity. However, dystocia, reduced birth weights, reduced neonatal survival, and reduced neonatal weight gain were noted. There are no adequate and well-controlled studies in pregnant women. Nicardipine hydrochloride capsules should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Studies in rats have shown significant concentrations of nicardipine in maternal milk following oral administration. For this reason it is recommended that women who wish to breastfeed should not take this drug.
Pediatric Use
Safety and efficacy in patients under the age of 18 have not been established.
Geriatric Use
Pharmacokinetic parameters did not differ between elderly hypertensive patients (≥65 years) and healthy controls after 1 week of nicardipine hydrochloride capsules treatment at 20 mg tid. Plasma nicardipine hydrochloride capsules concentrations in elderly hypertensive subjects were similar to plasma concentrations in healthy young adult subjects when nicardipine hydrochloride capsules were administered at doses of 10, 20, and 30 mg tid, suggesting that the pharmacokinetics of nicardipine hydrochloride capsules are similar in young and elderly hypertensive patients.
Clinical studies of nicardipine did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
Adverse Reactions/Side Effects
Angina
The incidence rates of adverse effects in anginal patients were derived from multicenter, controlled clinical trials. Following are the rates of adverse effects for nicardipine hydrochloride capsules (n=520) and placebo (n=310), respectively, that occurred in 0.4% of patients or more. These represent events considered probably drug-related by the investigator (except for certain cardiovascular events that were recorded in a different category). Where the frequency of adverse effects for nicardipine hydrochloride capsules and placebo is similar, causal relationship is uncertain. The only dose-related effects were pedal edema and increased angina.
Table 2
| Percent of Patients With Adverse Effects in Controlled Studies
(Incidence of Discontinuations Shown in Parentheses) |
||||
| ADVERSE EXPERIENCE
| NICARDIPINE HYDROCHLORIDE CAPSULES
(n= 520) | PLACEBO
(n= 310) |
||
| Pedal Edema | 7.1 | (0) | 0.3 | (0) |
| Dizziness | 6.9 | (1.2) | 0.6 | (0) |
| Headache | 6.4 | (0.6) | 2.6 | (0) |
| Asthenia | 5.8 | (0.4) | 2.6 | (0) |
| Flushing | 5.6 | (0.4) | 1.0 | (0) |
| Increased Angina | 5.6 | (3.5) | 4.2 | (1.9) |
| Palpitations | 3.3 | (0.4) | 0.0 | (0) |
| Nausea | 1.9 | (0) | 0.3 | (0) |
| Dyspepsia | 1.5 | (0.6) | 0.6 | (0.3) |
| Dry Mouth | 1.4 | (0) | 0.3 | (0) |
| Somnolence | 1.4 | (0) | 1.0 | (0) |
| Rash | 1.2 | (0.2) | 0.3 | (0) |
| Tachycardia | 1.2 | (0.2) | 0.6 | (0) |
| Myalgia | 1.0 | (0) | 0.0 | (0) |
| Other Edema | 1.0 | (0) | 0.0 | (0) |
| Paresthesia | 1.0 | (0.2) | 0.3 | (0) |
| Sustained Tachycardia | 0.8 | (0.6) | 0.0 | (0) |
| Syncope | 0.8 | (0.2) | 0.0 | (0) |
| Constipation | 0.6 | (0.2) | 0.6 | (0) |
| Dyspnea | 0.6 | (0) | 0.0 | (0) |
| Abnormal ECG | 0.6 | (0.6) | 0.0 | (0) |
| Malaise | 0.6 | (0) | 0.0 | (0) |
| Nervousness | 0.6 | (0) | 0.3 | (0) |
| Tremor | 0.6 | (0) | 0.0 | (0) |
Hypertension
The incidence rates of adverse effects in hypertensive patients were derived from multicenter, controlled clinical trials. Following are the rates of adverse effects for nicardipine hydrochloride capsules (n= 1390) and placebo (n= 211), respectively, that occurred in 0.4% of patients or more. These represent events considered probably drug-related by the investigator. Where the frequency of adverse effects for nicardipine hydrochloride capsules and placebo is similar, causal relationship is uncertain.
The only dose-related effect was pedal edema.
Table 3
| Percent of Patients with Adverse Effects in Controlled Studies
(Incidence of discontinuations shown in parentheses) |
||||
| ADVERSE EXPERIENCE
| NICARDIPINE HYDROCHLORIDE CAPSULES
(n = 1390) | PLACEBO
(n = 211) |
||
| Flushing | 9.7 | (2.1) | 2.8 | (0) |
| Headache | 8.2 | (2.6) | 4.7 | (0) |
| Pedal Edema | 8.0 | (1.8) | 0.9 | (0) |
| Asthenia | 4.2 | (1.7) | 0.5 | (0) |
| Palpitations | 4.1 | (1.0) | 0.0 | (0) |
| Dizziness | 4.0 | (1.8) | 0.0 | (0) |
| Tachycardia | 3.4 | (1.2) | 0.5 | (0) |
| Nausea | 2.2 | (0.9) | 0.9 | (0) |
| Somnolence | 1.1 | (0.1) | 0.0 | (0) |
| Dyspepsia | 0.8 | (0.3) | 0.5 | (0) |
| Insomnia | 0.6 | (0.1) | 0.0 | (0) |
| Malaise | 0.6 | (0.1) | 0.0 | (0) |
| Other Edema | 0.6 | (0.3) | 1.4 | (0) |
| Abnormal Dreams | 0.4 | (0) | 0.0 | (0) |
| Dry Mouth | 0.4 | (0.1) | 0.0 | (0) |
| Nocturia | 0.4 | (0) | 0.0 | (0) |
| Rash | 0.4 | (0.4) | 0.0 | (0) |
| Vomiting | 0.4 | (0.4) | 0.0 | (0) |
The following rare adverse events have been reported in clinical trials or the literature:
Body as a Whole: infection, allergic reaction
Cardiovascular: hypotension, postural hypotension, atypical chest pain, peripheral vascular disorder, ventricular extrasystoles, ventricular tachycardia
Digestive: sore throat, abnormal liver chemistries
Musculoskeletal: arthralgia
Nervous: hot flashes, vertigo, hyperkinesia, impotence, depression, confusion, anxiety
Respiratory: rhinitis, sinusitis
Special Senses: tinnitus, abnormal vision, blurred vision
Urogenital: increased urinary frequency
Overdosage
Based on results obtained in laboratory animals, overdosage may cause systemic hypotension, bradycardia (following initial tachycardia) and progressive atrioventricular conduction block. Reversible hepatic function abnormalities and sporadic focal hepatic necrosis were noted in some animal species receiving very large doses of nicardipine.
For treatment of overdose standard measures (for example, evacuation of gastric contents, elevation of extremities, attention to circulating fluid volume, and urine output) including monitoring of cardiac and respiratory functions should be implemented. The patient should be positioned so as to avoid cerebral anoxia. Frequent blood pressure determinations are essential. Vasopressors are clinically indicated for patients exhibiting profound hypotension. Intravenous calcium gluconate may help reverse the effects of calcium entry blockade.
Nicardipine Dosage and Administration
The dose should be individually titrated for each patient beginning with 20 mg three times daily. Doses in the range of 20 to 40 mg three times a day have been shown to be effective. At least 3 days should be allowed before increasing the nicardipine hydrochloride capsules dose to ensure achievement of steady-state plasma drug concentrations.
Concomitant Use With Other Antianginal Agents
- Sublingual NTG may be taken as required to abort acute anginal attacks during nicardipine hydrochloride capsules therapy.
- Prophylactic Nitrate Therapy: nicardipine hydrochloride capsules may be safely co-administered with short- and long-acting nitrates.
- Beta-blockers: Nicardipine hydrochloride capsules may be safely co-administered with beta- blockers (see Drug Interactions).
The dose of nicardipine hydrochloride capsules should be individually adjusted according to the blood pressure response beginning with 20 mg three times daily. The effective doses in clinical trials have ranged from 20 mg to 40 mg three times daily. The maximum blood pressure lowering effect occurs approximately 1 to 2 hours after dosing. To assess the adequacy of blood pressure response, the blood pressure should be measured at trough (8 hours after dosing). Because of the prominent peak effects of nicardipine, blood pressure should be measured 1 to 2 hours after dosing, particularly during initiation of therapy (see PRECAUTIONS: Blood Pressure, INDICATIONS AND USAGE, CLINICAL PHARMACOLOGY, Effects in Hypertension). At least 3 days should be allowed before increasing the nicardipine hydrochloride capsules dose to ensure achievement of steady-state plasma drug concentrations.
Concomitant Use With Other Antihypertensive Agents
- Diuretics: nicardipine hydrochloride capsules may be safety co-administered with thiazide diuretics.
- Beta-blockers: nicardipine hydrochloride capsules may be safely co-administered with beta-blocker (see PRECAUTIONS, Drug Interactions)
Renal Insufficiency
Although there is no evidence that nicardipine hydrochloride capsules impair renal function, careful dose titration beginning with 20 mg tid is advised (see PRECAUTIONS).
Hepatic Insufficiency
Nicardipine hydrochloride capsules should be administered cautiously in patients with severely impaired hepatic function. A suggested starting dose of 20 mg twice a day is advised with individual titration based on clinical findings maintaining the twice a day schedule (see PRECAUTIONS).
Congestive Heart Failure
Caution is advised when titrating nicardipine hydrochloride capsules dosage in patients with congestive heart failure (see WARNINGS).
How is Nicardipine supplied
They are available as:
Bottles of 90 with child-resistant closure: NDC 35573-457-85
Nicardipine hydrochloride 30 mg capsules are available in yellow powder filled in a hard gelatin capsule with a blue opaque cap and light blue opaque body, imprinted with "A42" on cap and body in black ink.
They are available as:
Bottles of 90 with child-resistant closure: NDC 35573-458-85
Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature].
Dispense contents in a tight, light-resistant container with a child-resistant closure.
The brands listed are trademarks of their respective owners and are not trademarks of Aavis Pharmaceuticals, Inc.
Manufactured for:
Burel Pharmaceuticals, LLC
Mason, OH 45040 USA
Code. L7064/00
Rev. 04/2023
| NICARDIPINE HYDROCHLORIDE
nicardipine hydrochloride capsule |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| NICARDIPINE HYDROCHLORIDE
nicardipine hydrochloride capsule |
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
|
||||||||||||||||||||||||||
| Labeler - Burel Pharmaceuticals, LLC (609436204) |






