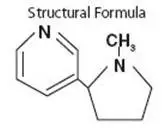
Drug Detail:Nicotrol ns (nasal, inhalation) (Nicotine (nasal, inhalation) [ nik-oh-teen ])
Drug Class: Smoking cessation agents
Nicotrol NS - Clinical Pharmacology
Pharmacodynamics
The cardiovascular effects of nicotine include peripheral vasoconstriction, tachycardia, and elevated blood pressure. Acute and chronic tolerance to nicotine develops from smoking tobacco or ingesting nicotine preparations. Acute tolerance (a reduction in response for a given dose) develops rapidly (less than 1 hour), but not at the same rate for different physiologic effects (skin temperature, heart rate, subjective effects). Withdrawal symptoms such as cigarette craving can be reduced in most individuals by plasma nicotine levels lower than those from smoking.
Withdrawal from nicotine in addicted individuals can be characterized by craving, nervousness, restlessness, irritability, mood lability, anxiety, drowsiness, sleep disturbances, impaired concentration, increased appetite, minor somatic complaints (headache, myalgia, constipation, fatigue), and weight gain. Nicotine toxicity is characterized by nausea, abdominal pain, vomiting, diarrhea, diaphoresis, flushing, dizziness, disturbed hearing and vision, confusion, weakness, palpitations, altered respiration and hypotension.
Both smoking and nicotine can increase circulating cortisol and catecholamines, and tolerance does not develop to the catecholamine-releasing effects of nicotine. Changes in the response to a concomitantly administered adrenergic agonist or antagonist should be watched for when nicotine intake is altered during NICOTROL NS therapy and/or smoking cessation (See PRECAUTIONS, Drug Interactions).
Warnings
Nicotine from any source can be toxic and addictive. Smoking causes lung disease, cancer, and heart disease and may adversely affect pregnant women or the fetus. For any smoker, with or without concomitant disease or pregnancy, the risk of nicotine replacement in a smoking cessation program should be weighed against the hazard of continued smoking, and the likelihood of achieving cessation of smoking without nicotine replacement.
Safety Note Concerning Children
The amounts of nicotine that are tolerated by adult smokers can produce signs and symptoms of poisoning and could prove fatal if NICOTROL NS is used or ingested by children or pets. Suspected nicotine poisoning in a child should be considered a medical emergency and treated immediately. A full bottle of NICOTROL NS contains 100 mg of nicotine, some of which will still be in the bottle when it is discarded. Therefore, patients should be cautioned to keep both used and unused containers of NICOTROL NS out of the reach of children and pets.
Precautions
Drug Interactions
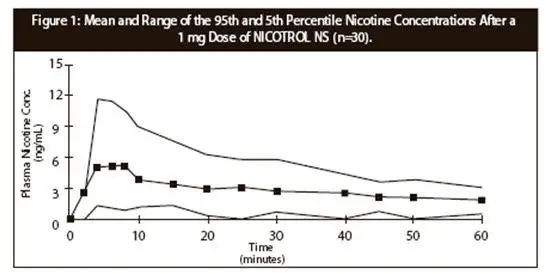
The extent of absorption and peak plasma concentration is slightly reduced in patients with the common cold/rhinitis. In addition, the time to peak concentration is prolonged. The use of a nasal vasoconstrictor such as xylometazoline in patients with rhinitis will further prolong the time to peak (See PHARMACOKINETICS). Smoking cessation, with or without nicotine replacement, may alter the pharmacokinetics of certain concomitant medications.
| May Require a Decrease in Dose at Cessation of Smoking | Possible Mechanism |
|---|---|
| Acetaminophen, caffeine, imipramine, oxazepam, pentazocine, propranolol, or other beta-blockers, theophylline | Deinduction of hepatic enzymes on smoking cessation. |
| Insulin | Increase of subcutaneous insulin absorption with smoking cessation. |
| Adrenergic antagonists (e.g. prazosin, labetalol) | Decrease in circulating catecholamines with smoking cessation. |
| May Require an Increase in Dose at Cessation of Smoking | Possible Mechanism |
| Adrenergic agonists (e.g. isoproterenol, phenylephrine) | Decrease in circulating catecholamines with smoking cessation. |
PREGNANCY
The harmful effects of cigarette smoking on maternal and fetal health are clearly established. These include low birth weight, an increased risk of spontaneous abortion, and increased perinatal mortality. The specific effects of NICOTROL NS on fetal development are unknown. Therefore pregnant smokers should be encouraged to attempt cessation using educational and behavioral interventions before using pharmacological approaches.
Spontaneous abortion during nicotine replacement therapy has been reported; as with smoking, nicotine as a contributing factor cannot be excluded.
NICOTROL NS should be used during pregnancy only if the likelihood of smoking cessation justifies the potential risk of using it by the pregnant patient, who might continue to smoke.
Adverse Reactions/Side Effects
Assessment of adverse events in the 730 patients who participated in controlled clinical trials is complicated by the occurrence of signs and symptoms of nicotine withdrawal in some patients and nicotine excess in others. The incidence of adverse events is confounded by the many minor complaints that smokers commonly have, by continued smoking by many patients and the local irritation from both active drug and the pepper placebo. No serious adverse events were reported during the trials.
Drug Abuse and Dependence
NICOTROL NS has a dependence potential intermediate between other nicotine-based therapies and cigarettes. This is the result of differences between cigarettes, NICOTROL NS, nicotine gum and nicotine patches in pharmacokinetic and dosing characteristics commonly associated with abuse and dependence. NICOTROL NS is distinct from other nicotine-based smoking cessation therapies in its greater speed of onset, greater capacity for self-titration of dose, and frequent and rapid fluctuations in plasma nicotine concentration.
Dependence on nicotine nasal spray occurred in the clinical trials. Feelings of dependency on the spray were reported by 32% of active spray users and 13% of placebo spray users. Such dependence may represent transference of tobacco-related nicotine dependence to NICOTROL NS.
Fifteen to 20% of patients used the active spray for longer periods than recommended (6 months to 1 year) and 5% used the spray at a higher dose than recommended. Some of these patients experienced anxiety about stopping the spray and some reported craving for the spray rather than for cigarettes.
Nicotrol NS Dosage and Administration
It is important that patients understand the instructions for use of NICOTROL NS, and have their questions answered. They should clearly understand the directions for using NICOTROL NS and safely disposing of the used container. They should be instructed to stop smoking completely when they begin using the product.
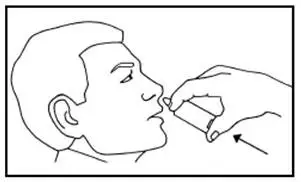
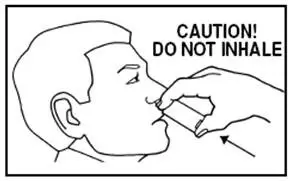
Patients should be instructed not to sniff, swallow or inhale through the nose as the spray is being administered. They should also be advised to administer the spray with the head tilted back slightly.
The dose of NICOTROL NS, should be individualized on the basis of each patient's nicotine dependence and the occurrence of symptoms of nicotine excess (See Individualization of Dosage).
Each actuation of NICOTROL NS delivers a metered 50 microliter spray containing 0.5 mg of nicotine. One dose is 1 mg of nicotine (2 sprays, one in each nostril).
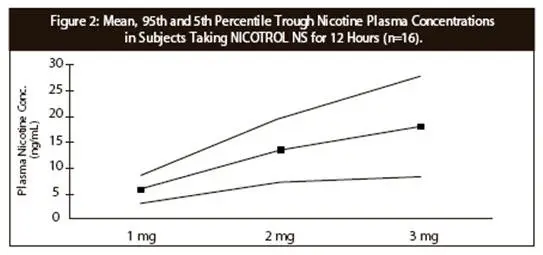
Patients should be started with 1 or 2 doses per hour, which may be increased up to a maximum recommended dose of 40 mg (80 sprays, somewhat less than 1/2 bottle) per day. For best results, patients should be encouraged to use at least the recommended minimum of 8 doses per day, as less is unlikely to be effective. In clinical trials, the patients who successfully quit smoking used the product heavily when nicotine withdrawal was at its peak, sometimes up to the recommended maximum of 40 doses per day ( in heavier smokers). Dosing recommendations are summarized in Table 4.
| Maximum Recommended Duration of Treatment | Recommended Doses per Hour | Maximum Doses per Hour | Maximum Doses per Day |
|---|---|---|---|
|
|||
| 3 months | 1–2* | 5 | 40 |
No tapering strategy has been shown to be optimal in clinical studies. Many patients simply stopped using the spray at their last clinic visit.
Recommended strategies for discontinuation of use include suggesting that patients: use only 1/2 a dose (1 spray) at a time, use the spray less frequently, keep a tally of daily usage, try to meet a steadily reducing usage target, skip a dose by not medicating every hour, or set a planned "quit date" for stopping use of the spray.
Individualization of Dosage
The success or failure of smoking cessation is influenced by the quality, intensity and frequency of supportive care. Patients are more likely to quit smoking if they are seen frequently and participate in formal smoking cessation programs.
The goal of NICOTROL NS therapy is complete abstinence. If a patient is unable to stop smoking by the fourth week of therapy, treatment should probably be discontinued.
Patients who fail to quit on any attempt may benefit from interventions to improve their chances for success on subsequent attempts. Patients who were unsuccessful should be counseled and should then probably be given a "therapy holiday" before the next attempt. A new quit attempt should be encouraged when conditions are more favorable.
Based on the clinical trials, a reasonable approach to assisting patients in their attempt to quit smoking is to begin initial treatment, using the recommended dosage (See DOSAGE AND ADMINISTRATION). Regular use of the spray during the first week of treatment may help patients adapt to the irritant effects of the spray. Dosage can then be adjusted in those subjects with signs or symptoms of nicotine withdrawal or excess. Patients who are successfully abstinent on NICOTROL NS should be treated at the selected dosage for up to 8 weeks, following which use of the spray should be discontinued over the next 4 to 6 weeks. Some patients may not require gradual reduction of dosage and may abruptly stop treatment successfully. Treatment with NICOTROL NS for longer periods has not been shown to improve outcome, and the safety of use for periods longer than 6 months has not been established.
The symptoms of nicotine withdrawal overlap those of nicotine excess (See Pharmacodynamics and ADVERSE REACTIONS sections). Since patients using NICOTROL NS may also smoke intermittently, it is sometimes difficult to determine if patients are experiencing nicotine withdrawal or nicotine excess. Controlled clinical trials of nicotine products suggest that palpitations, nausea and sweating are more often symptoms of nicotine excess, whereas anxiety, nervousness and irritability are more often symptoms of nicotine withdrawal.
| NICOTROL
nicotine spray, metered |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmacia & Upjohn Company LLC (618054084) |