Drug Detail:Nitromist (Nitroglycerin [ nye-troe-gli-ser-in ])
Drug Class: Antianginal agents Vasodilators
Highlights of Prescribing Information
NitroMist® (nitroglycerin) lingual aerosol
Initial U.S. Approval: 2006
Indications and Usage for NitroMist
NitroMist ® is a nitrate vasodilator indicated for acute relief of an attack or acute prophylaxis of angina pectoris due to coronary artery disease ( 1).
NitroMist Dosage and Administration
- At the onset of an attack, one metered spray or two metered sprays should be administered on or under the tongue. A spray may be repeated approximately every 5 minutes as needed (
2).
- Maximum of 3 metered sprays are recommended within a 15-minute period. If chest pain persists after a total of 3 sprays, prompt medical attention is recommended (
2).
- May be used prophylactically 5 minutes to 10 minutes before engaging in activities that might precipitate an acute attack ( 2).
Dosage Forms and Strengths
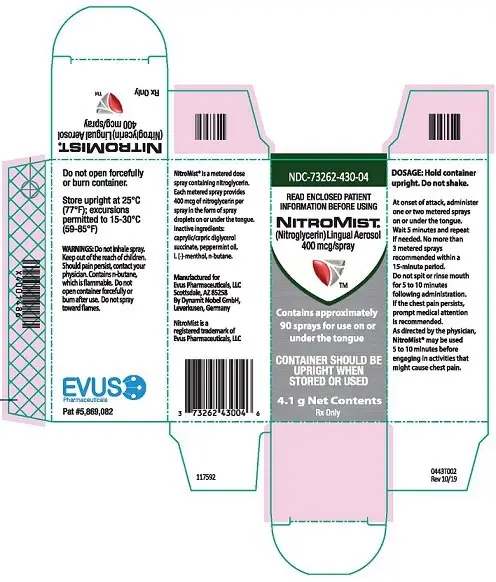
Lingual aerosol, 400 mcg per spray is available in either 230 metered sprays or 90 metered sprays per container ( 3)
Contraindications
- Use of a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5 inhibitors), such as sildenafil, vardenafil, and tadalafil (
4.1)
- Use of the soluble guanylate stimulator riociguat (
4.1)
- Severe anemia (
4.2)
- Increased intracranial pressure (
4.3)
- History of hypersensitivity to NitroMist or to other nitrates or nitrites ( 4.4)
Warnings and Precautions
- Tolerance: Excessive use may lead to tolerance ( 5.1).
Adverse Reactions/Side Effects
Most common adverse reactions are headache, flushing, hypotension, and syncope ( 6).
To report SUSPECTED ADVERSE REACTIONS, contact Evus Pharmaceuticals at 1-866-928-6180 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch/report.htm.
Drug Interactions
- PDE5 inhibitors: Concomitant use contraindicated (
4.1,
7.1)
- Soluble guanylate cyclase stimulator (sGS): Concomitant use contraindicated (
4.1,
7.1)
- Antihypertensives: Possible additive hypotensive effects (
7.2)
- Aspirin: increased nitroglycerin levels (
7.3)
- Tissue-type plasminogen activator (t-PA): decreased thrombolytic effect (
7.4)
- Heparin: anticoagulant effect of heparin may be reduced. Monitor APTT. (
7.5)
- Ergotamine: increased bioavailability of ergotamine. Avoid concomitant use. ( 7.6)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 9/2019
Related/similar drugs
amlodipine, lisinopril, metoprolol, losartan, aspirin, furosemide, carvedilolFull Prescribing Information
1. Indications and Usage for NitroMist
NitroMist is indicated for acute relief of an attack or acute prophylaxis of angina pectoris due to coronary artery disease.
2. NitroMist Dosage and Administration
2.3 Administration
During use the patient should rest, ideally in the sitting position. The container should be held vertically with the valve head uppermost and the spray orifice as close to the mouth as possible. The dose should preferably be sprayed into the mouth on or under the tongue by pressing the button firmly and the mouth should be closed immediately after each dose. THE SPRAY SHOULD NOT BE INHALED. Patients should be instructed to familiarize themselves with the position of the spray orifice, which can be identified by the finger rest on top of the valve, in order to facilitate orientation for administration at night.
- Do not shake container.
- Remove plastic cap.
- If this is the first time using the bottle, press actuator button 10 times to ensure proper dose priming (holding unit away from yourself and others).
- Hold container upright with forefinger on top of the actuator button.
- Open mouth and bring the container as close as possible.
- Press the actuator button firmly with forefinger to release spray(s) onto or under the tongue.
- Release button and close mouth. The medication should not be spit out or the mouth rinsed for 5 minutes to 10 minutes following administration.
- If a second administration is required to obtain relief, repeat steps 4, 5, and 6. No more than 3 metered sprays can be given within a 15-minute period.
- Replace plastic cover.
- If the product is not used for more than 6 weeks, then it can be adequately re-primed with 2 sprays.
The level of the liquid in the container should be periodically checked. While the container is in the upright position, if the liquid reaches the top or middle of the hole on the side of the container, one should order more. When the liquid reaches the bottom of the hole, the remaining doses will have less than label content.
3. Dosage Forms and Strengths
Lingual aerosol, 400 mcg per spray, is available in either 230 metered sprays or 90 metered sprays per container.
4. Contraindications
4.1 PDE5 Inhibitor Use
Administration of NitroMist is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5), as PDE5 inhibitors such as sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates. Do not use NitroMist in patients who are taking the soluble guanylate cyclase (sGC) stimulator riociguat. Concomitant use can cause hypotension. [see DRUG INTERACTIONS (7.1)].
5. Warnings and Precautions
5.1 Tolerance
Excessive use may lead to the development of tolerance. Only the smallest number of doses required for effective relief of the acute anginal attack should be used [see DOSAGE AND ADMINISTRATION (2)].
As tolerance to other forms of nitroglycerin develops, the effect of sublingual nitroglycerin on exercise tolerance, although still observable, is reduced.
7. Drug Interactions
7.1 PDE5 Inhibitors
Administration of NitroMist is contraindicated in patients who are using a selective inhibitor of cyclic guanosine monophosphate (cGMP)-specific phosphodiesterase type 5 (PDE5). PDE5 inhibitors such as sildenafil, vardenafil, and tadalafil have been shown to potentiate the hypotensive effects of organic nitrates. Do not use NitroMist in patients who are taking the soluble guanylate cyclase (sGC) stimulator riociguat. Concomitant use may result in severe hypotension, syncope, or myocardial ischemia.
The time course and dose dependence of this interaction have not been studied, and use within a few days of one another cannot be recommended. Appropriate supportive care for the severe hypotension has not been studied, but it seems reasonable to treat this as a nitrate overdose, with elevation of the extremities and with central volume expansion. The use of any form of nitroglycerin during the early days of acute myocardial infarction requires particular attention to hemodynamic monitoring and clinical status.
7.2 Antihypertensives
Patients receiving antihypertensive drugs, beta-adrenergic blockers, and nitrates should be observed for possible additive hypotensive effects. Marked orthostatic hypotension has been reported when calcium channel blockers and organic nitrates were used concomitantly.
Labetolol blunts the reflex tachycardia produced by nitroglycerin without preventing its hypotensive effects. If labetolol is used with nitroglycerin in patients with angina pectoris, additional hypotensive effects may occur.
7.3 Aspirin
Coadministration of aspirin and nitroglycerin has been reported to result in increased nitroglycerin maximum concentrations by as much as 67% and AUC by 73% when administered as a single dose. The vasodilatory and hemodynamic effects of nitroglycerin may be enhanced by concomitant administration of aspirin.
7.4 Tissue-type Plasminogen Activator (t-PA)
Intravenous administration of nitroglycerin decreases the thrombolytic effect of tissue-type plasminogen activator (t-PA). Plasma levels of t-PA are reduced when coadministered with nitroglycerin. Therefore, caution should be observed in patients receiving nitroglycerin during t-PA therapy.
7.5 Heparin
Intravenous nitroglycerin reduces the anticoagulant effect of heparin. Activated partial thromboplastin times (APTT) should be monitored in patients receiving heparin and intravenous nitroglycerin. It is not known if this effect occurs following single nitroglycerin doses.
7.6 Ergotamine
Oral administration of nitroglycerin markedly decreases the first-pass metabolism of dihydroergotamine and subsequently increases its oral bioavailability. Ergotamine is known to precipitate angina pectoris. Therefore, patients receiving sublingual nitroglycerin should avoid ergotamine and related drugs or be monitored for symptoms of ergotism if this is not possible.
17. Patient Counseling Information
17.5 Container information
The NitroMist bottle should not be forcefully opened. Because NitroMist contains a highly flammable propellant (butane), do not have the container burned after use and do not spray directly towards flames.
While the container is in the upright position, if the liquid reaches the top to middle of the hole on the side of the container, a new supply should be obtained. When the liquid reaches the bottom of the hole, the remaining doses will have less than label content .
Manufactured for Evus Pharmaceuticals, LLC

Scottsdale, AZ 85258 USA
by Dynamit Nobel GmbH, Leverkusen, Germany
Marketed and Distributed by: Evus Pharmaceuticals, LLC
Scottsdale, AZ 85258 USA
NitroMist is a registered trademark of Evus Pharmaceuticals
141110
143F003
Revised 09/2019
PRINCIPAL DISPLAY PANEL
NDC 73262-430-08
NITROMIST
(Nitroglycerin) Lingual Aerosol
400 mcg/spray
contains approximately
230 sprays for use on or
under the tongue
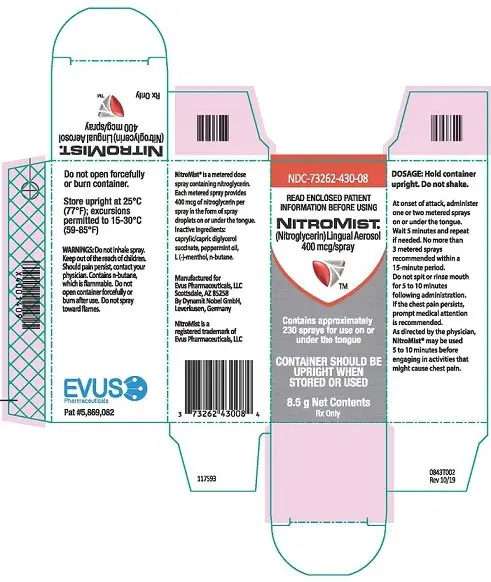
PRINCIPAL DISPLAY PANEL
NDC 73262-430-08
NITROMIST
(Nitroglycerin) Lingual Aerosol
400 mcg/spray
contains approximately
230 sprays for use on or
under the tongue

| NITROMIST
nitroglycerin aerosol, metered |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Evus Pharmaceuticals, LLC (117137572) |