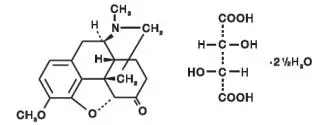
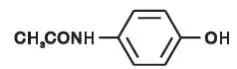
Drug Detail:Norco (Acetaminophen and hydrocodone [ a-seet-a-min-oh-fen-and-hye-droe-koe-done ])
Drug Class: Narcotic analgesic combinations
Indications and Usage for Norco
NORCO is indicated for the management of pain severe enough to require an opioid analgesic and for which alternative treatments are inadequate.
Limitations of Use:
Because of the risks of addiction, abuse, and misuse, with opioids, even at recommended doses [see WARNINGS], reserve NORCO for use in patients for whom alternative treatment options (e.g., non-opioid analgesics):
- have not been tolerated, or are not expected to be tolerated
- have not provided adequate analgesia, or are not expected to provide adequate analgesia
Warnings
Addiction, Abuse, and Misuse
NORCO contains hydrocodone, a Schedule II controlled substance. As an opioid, NORCO exposes users to the risks of addiction, abuse, and misuse [see DRUG ABUSE AND DEPENDENCE].
Although the risk of addiction in any individual is unknown, it can occur in patients appropriately prescribed NORCO. Addiction can occur at recommended dosages and if the drug is misused or abused.
Assess each patient’s risk for opioid addiction, abuse, or misuse prior to prescribing NORCO, and monitor all patients receiving NORCO for the development of these behaviors and conditions. Risks are increased in patients with a personal or family history of substance abuse (including drug or alcohol abuse or addiction) or mental illness (e.g., major depression). The potential for these risks should not, however, prevent the proper management of pain in any given patient. Patients at increased risk may be prescribed opioids such as NORCO, but use in such patients necessitates intensive counseling about the risks and proper use of NORCO along with intensive monitoring for signs of addiction, abuse, and misuse. Consider prescribing naloxone for the emergency treatment of opioid overdose [see WARNINGS; Life-Threatening Respiratory Depression, DOSAGE AND ADMINSTRATION; Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose].
Opioids are sought by drug abusers and people with addiction disorders and are subject to criminal diversion. Consider these risks when prescribing or dispensing NORCO. Strategies to reduce these risks include prescribing the drug in the smallest appropriate quantity and advising the patient on the proper disposal of unused drug [see PRECAUTIONS; Information for Patients]. Contact local state professional licensing board or state-controlled substances authority for information on how to prevent and detect abuse or diversion of this product.
Opioid Analgesic Risk Evaluation and Mitigation Strategy (REMS)
To ensure that the benefits of opioid analgesics outweigh the risks of addiction, abuse, and misuse, the Food and Drug Administration (FDA) has required a Risk Evaluation and Mitigation Strategy (REMS) for these products. Under the requirements of the REMS, drug companies with approved opioid analgesic products must make REMS-compliant education programs available to healthcare providers. Healthcare providers are strongly encouraged to do all of the following:
- Complete a REMS-compliant education program offered by an accredited provider of continuing education (CE) or another education program that includes all the elements of the FDA Education Blueprint for Healthcare Providers Involved in the Management or Support of Patients with Pain.
- Discuss the safe use, serious risks, and proper storage and disposal of opioid analgesics with patients and/or their caregivers every time these medicines are prescribed. The Patient Counseling Guide (PCG) can be obtained at this link: www.fda.gov/OpioidAnalgesicREMSPCG.
- Emphasize to patients and their caregivers the importance of reading the Medication Guide that they will receive from their pharmacist every time an opioid analgesic is dispensed to them.
- Consider using other tools to improve patient, household, and community safety, such as patient-prescriber agreements that reinforce patient-prescriber responsibilities.
To obtain further information on the opioid analgesic REMS and for a list of accredited REMS CME/CE, call 1-800-503-0784, or log on to www.opioidanalgesicrems.com. The FDA Blueprint can be found at www.fda.gov/OpioidAnalgesicREMSBlueprint.
Life-Threatening Respiratory Depression
Serious, life-threatening, or fatal respiratory depression has been reported with the use of opioids, even when used as recommended. Respiratory depression, if not immediately recognized and treated, may lead to respiratory arrest and death. Management of respiratory depression may include close observation, supportive measures, and use of opioid antagonists, depending on the patient’s clinical status [see OVERDOSAGE]. Carbon dioxide (CO2) retention from opioid-induced respiratory depression can exacerbate the sedating effects of opioids.
While serious, life-threatening, or fatal respiratory depression can occur at any time during the use of NORCO, the risk is greatest during the initiation of therapy or following a dosage increase. Monitor patients closely for respiratory depression, especially within the first 24-72 hours of initiating therapy with and following dosage increases of NORCO.
To reduce the risk of respiratory depression, proper dosing and titration of NORCO is essential [see DOSAGE AND ADMINISTRATION]. Overestimating the NORCO dosage when converting patients from another opioid product can result in a fatal overdose.
Accidental ingestion of even one dose of NORCO, especially by children, can result in respiratory depression and death due to an overdose of NORCO.
Educate patients and caregivers on how to recognize respiratory depression and emphasize the importance of calling 911 or getting emergency medical help right away in the event of a known or suspected overdose [see PRECAUTIONS; Information for Patients].
Opioids can cause sleep-related breathing disorders including central sleep apnea (CSA) and sleep-related hypoxemia. Opioid use increases the risk of CSA in a dose-dependent fashion. In patients who present with CSA, consider decreasing the opioid dosage using best practices for opioid taper [see DOSAGE AND ADMINISTRATION].
Risks from Concomitant Use with Benzodiazepines or Other CNS Depressants
Profound sedation, respiratory depression, coma, and death may result from the concomitant use of NORCO Tablets with benzodiazepines or other CNS depressants (e.g., non-benzodiazepine sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol). Because of these risks, reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate.
Observational studies have demonstrated that concomitant use of opioid analgesics and benzodiazepines increases the risk of drug-related mortality compared to use of opioid analgesics alone. Because of similar pharmacological properties, it is reasonable to expect similar risk with the concomitant use of other CNS depressant drugs with opioid analgesics [see PRECAUTIONS; Drug Interactions].
If the decision is made to prescribe a benzodiazepine or other CNS depressant concomitantly with an opioid analgesic, prescribe the lowest effective dosages and minimum durations of concomitant use. In patients already receiving an opioid analgesic, prescribe a lower initial dose of the benzodiazepine or other CNS depressant than indicated in the absence of an opioid, and titrate based on clinical response. If an opioid analgesic is initiated in a patient already taking a benzodiazepine or other CNS depressant, prescribe a lower initial dose of the opioid analgesic, and titrate based on clinical response. Follow patients closely for signs and symptoms of respiratory depression and sedation.
If concomitant use is warranted, consider prescribing naloxone for the emergency treatment of opioid overdose [see WARNINGS; Life-Threatening Respiratory Depression, DOSAGE AND ADMINISTRATION; Patient Access to Naloxone for the Emergency Treatment of Opioid Overdose].
Advise both patients and caregivers about the risks of respiratory depression and sedation when NORCO Tablets are used with benzodiazepines or other CNS depressants (including alcohol and illicit drugs). Advise patients not to drive or operate heavy machinery until the effects of concomitant use of the benzodiazepine or other CNS depressant have been determined. Screen patients for risk of substance use disorders, including opioid abuse and misuse, and warn them of the risk for overdose and death associated with the use of additional CNS depressants including alcohol and illicit drugs [see PRECAUTIONS; Drug Interactions; Information for Patients].
Withdrawal
Do not abruptly discontinue NORCO Tablets in a patient physically dependent on opioids. When discontinuing NORCO Tablets in a physically dependent patient, gradually taper the dosage. Rapid tapering of NORCO Tablets in a patient physically dependent on opioids may lead to a withdrawal syndrome and return of pain [see DOSAGE AND ADMINISTRATION, DRUG ABUSE AND DEPENDENCE].
Additionally, avoid the use of mixed agonist/antagonist (e.g., pentazocine, nalbuphine, and butorphanol) or partial agonist (e.g., buprenorphine) analgesics in patients who are receiving a full opioid agonist analgesic, including NORCO Tablets. In these patients, mixed agonist/antagonist and partial agonist analgesics may reduce the analgesic effect and/or precipitate withdrawal symptoms [see PRECAUTIONS; Drug Interactions].
Precautions
Risks of Driving and Operating Machinery
NORCO Tablets may impair the mental or physical abilities needed to perform potentially hazardous activities such as driving a car or operating machinery. Warn patients not to drive or operate dangerous machinery unless they are tolerant to the effects of NORCO Tablets and know how they will react to the medication [see PRECAUTIONS; Information for Patients].
Information for Patients
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Drug Interactions
Inhibitors of CYP3A4 and CYP2D6
The concomitant use of NORCO and CYP3A4 inhibitors, such as macrolide antibiotics (e.g., erythromycin), azole-antifungal agents (e.g. ketoconazole), and protease inhibitors (e.g., ritonavir), can increase the plasma concentration of the hydrocodone from NORCO Tablets, resulting in increased or prolonged opioid effects. These effects could be more pronounced with concomitant use of NORCO and both CYP3A4 and CYP2D6 inhibitors, particularly when an inhibitor is added after a stable dose of NORCO is achieved [see WARNINGS].
After stopping a CYP3A4 inhibitor, as the effects of the inhibitor decline, the NORCO plasma concentration will decrease [see CLINICAL PHARMACOLOGY], resulting in decreased opioid efficacy or a withdrawal syndrome in patients who had developed physical dependence to NORCO.
If concomitant use is necessary, consider dosage reduction of NORCO until stable drug effects are achieved. Follow patients for respiratory depression and sedation at frequent intervals. If a CYP3A4 inhibitor is discontinued, consider increasing the NORCO dosage until stable drug effects are achieved. Follow for signs or symptoms of opioid withdrawal.
Inducers of CYP3A4
The concomitant use of NORCO and CYP3A4 inducers, such as rifampin, carbamazepine, and phenytoin, can decrease the plasma concentration of NORCO [see CLINICAL PHARMACOLOGY], resulting in decreased efficacy or onset of a withdrawal syndrome in patients who have developed physical dependence to NORCO [see WARNINGS].
After stopping a CYP3A4 inducer, as the effects of the inducer decline, the NORCO plasma concentration will increase [see CLINICAL PHARMACOLOGY], which could increase or prolong both the therapeutic effects and adverse reactions, and may cause serious respiratory depression.
If concomitant use is necessary, consider increasing the NORCO dosage until stable drug effects are achieved. Follow the patient for signs and symptoms of opioid withdrawal. If a CYP3A4 inducer is discontinued, consider NORCO dosage reduction and follow for signs of respiratory depression.
Benzodiazepines and Other CNS Depressants
Clinical Impact: Due to additive pharmacologic effect, the concomitant use of benzodiazepines or other CNS depressants, including alcohol, can increase the risk of hypotension, respiratory depression, profound sedation, coma, and death.
Intervention: Reserve concomitant prescribing of these drugs for use in patients for whom alternative treatment options are inadequate. Limit dosages and durations to the minimum required. Follow patients closely for signs of respiratory depression and sedation. If concomitant use is warranted, consider prescribing naloxone for the emergency treatment of opioid overdose [see WARNINGS].
Examples: Benzodiazepines and other sedatives/hypnotics, anxiolytics, tranquilizers, muscle relaxants, general anesthetics, antipsychotics, other opioids, alcohol.
Serotonergic Drugs
The concomitant use of opioids with other drugs that affect the serotonergic neurotransmitter system, such as selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), triptans, 5-HT3 receptor antagonists, drugs that affect the serotonin neurotransmitter system (e.g., mirtazapine, trazodone, tramadol), certain muscle relaxants (i.e., cyclobenzaprine, metaxalone), and monoamine oxidase (MAO) inhibitors (those intended to treat psychiatric disorders and also others, such as linezolid and intravenous methylene blue), has resulted in serotonin syndrome [see PRECAUTIONS; Information for Patients].
If concomitant use is warranted, carefully follow the patient, particularly during treatment initiation and dose adjustment. Discontinue NORCO if serotonin syndrome is suspected.
Monoamine Oxidase Inhibitors (MAOIs)
The concomitant use of opioids and MAOIs, such as phenelzine, tranylcypromine, or linezolid, may manifest as serotonin syndrome, or opioid toxicity (e.g., respiratory depression, coma) [see WARNINGS].
The use of NORCO is not recommended for patients taking MAOIs or within 14 days of stopping such treatment.
If urgent use of an opioid is necessary, use test doses and frequent titration of small doses to treat pain while closely monitoring blood pressure and signs and symptoms of CNS and respiratory depression.
Mixed Agonist/Antagonist and Partial Agonist Opioid Analgesics
The concomitant use of opioids with other opioid analgesics, such as butorphanol, nalbuphine, pentazocine, may reduce the analgesic effect of NORCO and/or precipitate withdrawal symptoms.
Advise patient to avoid concomitant use of these drugs.
Muscle Relaxants
Clinical Impact: NORCO may enhance the neuromuscular blocking action of skeletal muscle relaxants and produce an increased degree of respiratory depression.
Intervention: Monitor patients for signs of respiratory depression that may be greater than otherwise expected and decrease the dosage of NORCO and/or the muscle relaxant as necessary. Due to the risk of respiratory depression with concomitant use of skeletal muscle relaxants and opioids, consider prescribing naloxone for the emergency treatment of opioid overdose [see WARNINGS].
Examples: cyclobenzaprine, metaxalone
Diuretics
Opioids can reduce the efficacy of diuretics by inducing the release of antidiuretic hormone.
If concomitant use is warranted, follow patients for signs of diminished diuresis and/or effects on blood pressure and increase the dosage of the diuretic as needed.
Anticholinergic Drugs
The concomitant use of anticholinergic drugs may increase risk of urinary retention and/or severe constipation, which may lead to paralytic ileus.
If concomitant use is warranted, follow patients for signs and symptoms of urinary retention or reduced gastric motility when NORCO Tablets are used concomitantly with anticholinergic drugs.
Pregnancy
Teratogenic Effects
There are no adequate and well-controlled studies in pregnant women. NORCO Tablets should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
Hydrocodone is present in human milk.
The developmental and health benefits of breast-feeding should be considered along with the mother’s clinical need for NORCO and any potential adverse effects on the breastfed infant from NORCO or from the underlying maternal condition.
Infants exposed to NORCO through breast milk should be monitored for excess sedation and respiratory depression. Withdrawal symptoms can occur in breastfed infants when maternal administration of an opioid analgesic is stopped, or when breast-feeding is stopped.
Adverse Reactions/Side Effects
The following adverse reactions have been identified during post approval use of NORCO. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
The most frequently reported adverse reactions are light-headedness, dizziness, sedation, nausea and vomiting. Other adverse reactions include:
Central Nervous System – Drowsiness, mental clouding, lethargy, impairment of mental and physical performance, anxiety, fear, dysphoria, psychological dependence, mood changes.
Gastrointestinal System – Constipation.
Genitourinary System – Ureteral spasm, spasm of vesical sphincters, and urinary retention.
Special Senses – Cases of hearing impairment or permanent loss have been reported predominantly in patients with chronic overdose.
Dermatological – Skin rash, pruritus, Stevens-Johnson syndrome, toxic epidermal necrolysis, allergic reactions
Hematological – Thrombocytopenia, agranulocytosis.
-
Serotonin syndrome: Cases of serotonin syndrome, a potentially life-threatening condition, have been reported during concomitant use of opioids with serotonergic drugs.
-
Adrenal insufficiency: Cases of adrenal insufficiency have been reported with opioid use, more often following greater than one month of use.
-
Anaphylaxis: Anaphylaxis has been reported with ingredients contained in NORCO.
- Androgen deficiency: Cases of androgen deficiency have occurred with chronic use of opioids [see CLINICAL PHARMACOLOGY].
Overdosage
Following an acute overdosage, toxicity may result from hydrocodone or acetaminophen.
Clinical Presentation
Acute overdosage with NORCO can be manifested by respiratory depression, somnolence progressing to stupor or coma, skeletal muscle flaccidity, cold and clammy skin, constricted pupils, and, in some cases, pulmonary edema, bradycardia, hypotension, partial or complete airway obstruction, atypical snoring, and death. Marked mydriasis rather than miosis may be seen with hypoxia in overdose situations.
Acetaminophen
Dose-dependent, potentially fatal hepatic necrosis is the most serious adverse effect of acetaminophen overdosage. Renal tubular necrosis, hypoglycemic coma and coagulation defects may also occur.
Early symptoms following a potentially hepatotoxic overdose may include: nausea, vomiting, diaphoresis and general malaise. Clinical and laboratory evidence of hepatic toxicity may not be apparent until 48 to 72 hours post-ingestion.
Treatment of Overdose
Hydrocodone
In case of overdose, priorities are the re-establishment of a patent and protected airway and institution of assisted or controlled ventilation, if needed. Employ other supportive measures (including oxygen and vasopressors) in the management of circulatory shock and pulmonary edema as indicated. Cardiac arrest or arrhythmias will require advanced life-support techniques.
Opioid antagonists, such as naloxone, are specific antidotes to respiratory depression resulting from opioid overdose. For clinically significant respiratory or circulatory depression secondary to NORCO overdose, administer an opioid antagonist.
Because the duration of opioid reversal is expected to be less than the duration of action of NORCO in NORCO Tablets, carefully monitor the patient until spontaneous respiration is reliably re-established. If the response to an opioid antagonist is suboptimal or only brief in nature, administer additional antagonist as directed by the product’s prescribing information.
In an individual physically dependent on opioids, administration of the recommended usual dosage of the antagonist will precipitate an acute withdrawal syndrome. The severity of the withdrawal symptoms experienced will depend on the degree of physical dependence and the dose of the antagonist administered. If a decision is made to treat serious respiratory depression in the physically dependent patient, administration of the antagonist should be initiated with care and by titration with smaller than usual doses of the antagonist.
Acetaminophen
Gastric decontamination with activated charcoal should be administered just prior to N-acetylcysteine (NAC) to decrease systemic absorption if acetaminophen ingestion is known or suspected to have occurred within a few hours of presentation. Serum acetaminophen levels should be obtained immediately if the patient presents 4 hours or more after ingestion to assess potential risk of hepatotoxicity; acetaminophen levels drawn less than 4 hours post-ingestion may be misleading. To obtain the best possible outcome, NAC should be administered as soon as possible where impending or evolving liver injury is suspected. Intravenous NAC may be administered when circumstances preclude oral administration.
Vigorous supportive therapy is required in severe intoxication. Procedures to limit the continuing absorption of the drug must be readily performed since the hepatic injury is dose dependent and occurs early in the course of intoxication.
Medication Guide
| Medication Guide
NORCO® (nor koe’) (Hydrocodone Bitartrate and Acetaminophen Tablets, USP) CII |
|
NORCO® is:
|
|
Important information about NORCO:
|
|
Do not take NORCO if you have:
|
|
| Before taking NORCO, tell your healthcare provider if you have a history of: | |
| ● head injury, seizures | ● liver, kidney, thyroid problems |
| ● problems urinating | ● pancreas or gallbladder problems |
| ● abuse of street or prescription drugs, alcohol addiction, opioid overdose, or mental health problems. |
|
Tell your healthcare provider if you are:
|
|
When taking NORCO:
|
|
While taking NORCO DO NOT:
|
|
The possible side effects of NORCO:
|
|
| For more information call Allergan at 1-800-678-1605 Distributed by: Allergan USA, Inc. Madison, NJ 07940 ©2021 Allergan. All rights reserved. NORCO® is a registered trademark of Allergan Sales, LLC. |
|
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 03/2021
v3.0MG6002
| NORCO
hydrocodone bitartrate and acetaminophen tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| NORCO
hydrocodone bitartrate and acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NORCO
hydrocodone bitartrate and acetaminophen tablet |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Allergan, Inc. (144796497) |