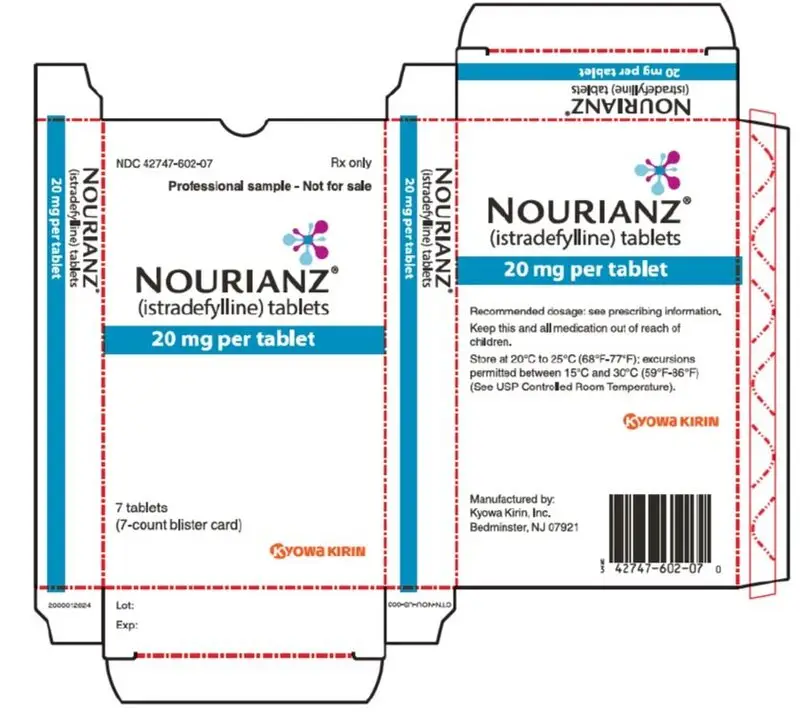
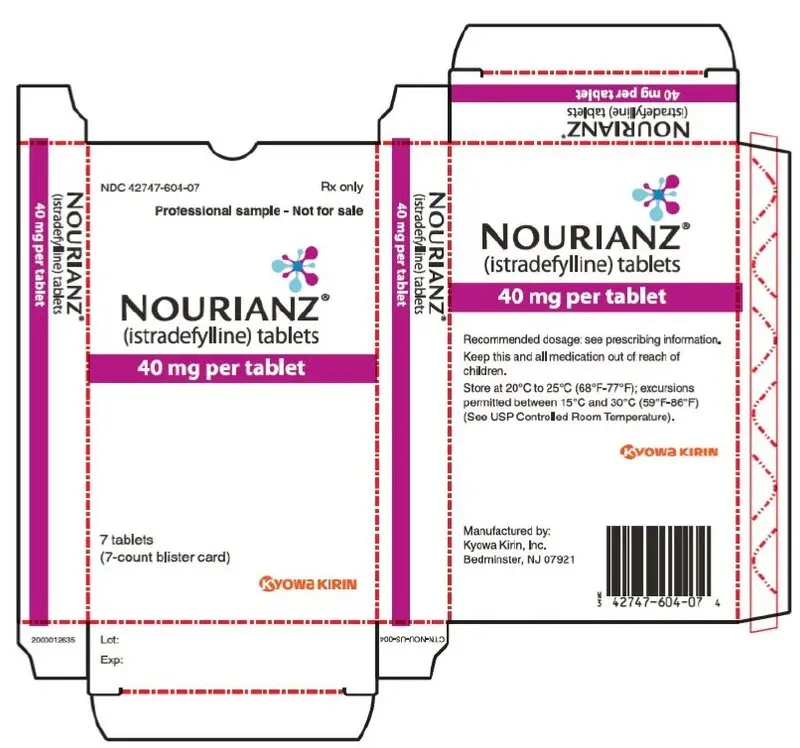
Drug Detail:Nourianz (Istradefylline [ is-tra-def-i-lin ])
Drug Class: Miscellaneous antiparkinson agents
Highlights of Prescribing Information
NOURIANZ® (istradefylline) tablets, for oral use
Initial U.S. Approval: 2019
Indications and Usage for Nourianz
NOURIANZ is an adenosine receptor antagonist indicated as adjunctive treatment to levodopa/carbidopa in adult patients with Parkinson's disease (PD) experiencing "off" episodes (1).
Nourianz Dosage and Administration
- The recommended dosage is 20 mg orally once daily. The dosage may be increased to a maximum of 40 mg once daily (2.1).
- May be taken with or without food (2.1).
- Patients with hepatic impairment: Maximum recommended dosage with moderate hepatic impairment is 20 mg once daily; use of NOURIANZ in patients with severe hepatic impairment should be avoided (2.4, 8.7).
- Patients who smoke 20 or more cigarettes per day (or the equivalent of another tobacco product): Recommended dosage is 40 mg once daily (2.5, 8.8).
Dosage Forms and Strengths
Tablets: 20 mg and 40 mg (3).
Contraindications
None (4).
Warnings and Precautions
- Dyskinesia: Monitor patients for dyskinesia or exacerbation of existing dyskinesia (5.1).
- Hallucinations / Psychotic Behavior: Consider dosage reduction or stopping NOURIANZ if occurs (5.2).
- Impulse Control / Compulsive Behaviors: Consider dosage reduction or stopping NOURIANZ if occurs (5.3).
Adverse Reactions/Side Effects
The most common adverse reactions (at least 5% and more frequent than placebo) were dyskinesia, dizziness, constipation, nausea, hallucination, and insomnia (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Kyowa Kirin Inc. at 1-844-768-3544 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong CYP 3A4 inhibitors: Recommended maximum dosage with concomitant use is 20 mg once daily (2.2, 7.1).
- Strong CYP 3A4 inducers: Avoid use (2.3, 7.1).
Use In Specific Populations
- Pregnancy: Based on animal data, may cause fetal harm (8.1).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 5/2020
Full Prescribing Information
1. Indications and Usage for Nourianz
NOURIANZ is indicated as adjunctive treatment to levodopa/carbidopa in adult patients with Parkinson's disease (PD) experiencing "off" episodes.
2. Nourianz Dosage and Administration
2.1 Dosing Information
The recommended dosage of NOURIANZ is 20 mg administered orally once daily. The dosage may be increased to a maximum of 40 mg once daily, based on individual need and tolerability. Initial dose titration is not required.
NOURIANZ can be taken with or without food [see Clinical Pharmacology (12.3)].
2.2 Dosage Adjustment with Strong CYP 3A4 Inhibitors
The maximum recommended dosage of NOURIANZ with concomitant use of strong CYP3A4 inhibitors is 20 mg once daily [see Drug Interactions (7.1)].
2.3 Dosing with Strong CYP 3A4 Inducers
Avoid use of NOURIANZ with strong CYP3A4 inducers [see Drug Interactions (7.1)].
2.4 Dosage Adjustment in Patients with Hepatic Impairment
The maximum recommended dosage of NOURIANZ in patients with moderate hepatic impairment (Child-Pugh B) is 20 mg once daily. Closely monitor patients with moderate hepatic impairment for adverse reactions when on NOURIANZ treatment [see Adverse Reactions (6.1)]. Avoid use of NOURIANZ in patients with severe hepatic impairment (Child-Pugh C) [see Use in Specific Populations (8.7)].
3. Dosage Forms and Strengths
- 20 mg tablets: Peach-colored, pillow-shaped, film-coated tablets with "20" debossed on one side.
- 40 mg tablets: Peach-colored, almond-shaped, film-coated tablets with "40" debossed on one side.
5. Warnings and Precautions
5.1 Dyskinesia
NOURIANZ in combination with levodopa may cause dyskinesia or exacerbate pre-existing dyskinesia.
In controlled clinical trials (Studies 1, 2, 3, and 4) [see Clinical Studies (14)], the incidence of dyskinesia was 15% for NOURIANZ 20 mg, 17% for NOURIANZ 40 mg, and 8% for placebo, in combination with levodopa. One percent of patients treated with either NOURIANZ 20 mg or 40 mg discontinued treatment because of dyskinesia, compared to 0% for placebo.
5.2 Hallucinations / Psychotic Behavior
Because of the potential risk of exacerbating psychosis, patients with a major psychotic disorder should not be treated with NOURIANZ. Consider dosage reduction or discontinuation if a patient develops hallucinations or psychotic behaviors while taking NOURIANZ.
In controlled clinical trials (Studies 1, 2, 3, and 4) [see Clinical Studies (14)], the incidence of hallucinations was 2% for NOURIANZ 20 mg, 6% for NOURIANZ 40 mg, and 3% for placebo. In patients treated with NOURIANZ 40 mg, 1% discontinued because of hallucinations, compared to 0% for placebo and 0% for patients treated with NOURIANZ 20 mg. The incidence of "abnormal thinking and behavior" (paranoid ideation, delusions, confusion, mania, disorientation, aggressive behavior, agitation, or delirium) reported as an adverse reaction was 1% for NOURIANZ 20 mg, 2% for NOURIANZ 40 mg, and 1% for placebo.
5.3 Impulse Control / Compulsive Behaviors
Patients treated with NOURIANZ and one or more medication(s) for the treatment of Parkinson's disease (including levodopa) may experience intense urges to gamble, increased sexual urges, intense urges to spend money, binge or compulsive eating, and/or other intense urges, and the inability to control these urges. In controlled clinical trials (Studies 1, 2, 3 and 4) [see Clinical Studies (14)],one patient treated with NOURIANZ 40 mg was reported to have impulse control disorder, compared to no patient on placebo or NOURIANZ 20 mg.
In some postmarketing cases, these urges were reported to have stopped when the dose was reduced, or the medication was discontinued. Because patients may not recognize these behaviors as abnormal, it is important for prescribers to specifically ask patients or their caregivers about the development of new or increased gambling urges, sexual urges, uncontrolled spending, binge or compulsive eating, or other urges while being treated with NOURIANZ. Consider dose reduction or discontinuation if a patient develops such urges while taking NOURIANZ [see Adverse Reactions (6.2)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Dyskinesia [see Warnings and Precautions (5.1)]
- Hallucinations / Psychotic Behavior [see Warnings and Precautions (5.2)]
- Impulse Control / Compulsive Behaviors [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of NOURIANZ was evaluated in 734 patients with Parkinson's disease (PD) taking a stable dose of levodopa and a DOPA decarboxylase inhibitor, with or without other PD medications, in four randomized, multicenter, double-blind, placebo-controlled trials 12 weeks in duration (Studies 1, 2, 3 and 4) [see Clinical Studies (14)]. Of the patient population exposed to NOURIANZ, 50% were male, 32% White, 67% Asian, and the mean age was 65 years (range: 33 to 84 years). Of these patients, 356 received NOURIANZ 20 mg and 378 received NOURIANZ 40 mg.
6.2 Postmarketing Experience
The following adverse reaction has been identified during post approval use of istradefylline outside of the United States. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure: increased libido.
8. Use In Specific Populations
8.5 Geriatric Use
No adjustment of NOURIANZ dosage is recommended on the basis of age. Of the total number of PD patients who received NOURIANZ in clinical trials, 53% were ≥65 years and 13% were ≥75 years of age. No overall differences in effectiveness were observed between these patients and younger patients.
8.6 Renal Impairment
No adjustment of NOURIANZ dosage is needed in patients with mild renal impairment (estimated creatinine clearance (CrCL) by Cockcroft-Gault equation: 60-89 mL/min), moderate renal impairment (CrCL 30-59 mL/min), or severe renal impairment (CrCL 15-29 mL/min). NOURIANZ has not been evaluated in patients with end-stage renal disease (ESRD) (CrCL <15 mL/min) or ESRD requiring hemodialysis [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No adjustment of NOURIANZ dosage is needed in patients with mild hepatic impairment (Child-Pugh Class A).
In patients with moderate hepatic impairment (Child-Pugh B), the steady-state exposures (AUC0-24h) were predicted to be 3.3-fold higher than in healthy subjects, based on the estimated mean terminal half-life. Therefore, the maximum recommended dosage of NOURIANZ in patients with moderate hepatic impairment (Child-Pugh B) is 20 mg once daily [see Clinical Pharmacology (12.3)]. Closely monitor patients with moderate hepatic impairment for adverse events when on NOURIANZ treatment [see Adverse Reactions (6.1)].
NOURIANZ has not been studied in patients with severe hepatic impairment (Child-Pugh Class C). Avoid use of NOURIANZ in patients with severe hepatic impairment [see Clinical Pharmacology (12.3)].
8.8 Tobacco Smokers
Tobacco smoking decreased NOURIANZ steady-state systemic exposures by 38% to 54% [see Clinical Pharmacology (12.3)], which may decrease efficacy. Therefore, the recommended NOURIANZ dosage in patients who smoke 20 or more cigarettes per day (or the equivalent amount of another tobacco product) is 40 mg once daily.
10. Overdosage
10.1 Human Experience
There is limited clinical experience regarding human overdosage with NOURIANZ. In clinical trials, one patient took 6 tablets (120 mg, 3 times the maximum recommended dosage) of istradefylline with alcoholic beverages and developed hallucinations, agitation, and worsening dyskinesia.
10.2 Management of Overdose
There are no known specific antidotes for NOURIANZ nor any specific treatment for istradefylline overdose. If an overdose occurs, NOURIANZ treatment should be discontinued and supportive treatment should be administered as clinically indicated. Consider the long terminal half-life of istradefylline (about 83 hours) and the possibility of multiple drug involvement.
Consult a Certified Poison Control Center for up-to-date guidance and advice.
11. Nourianz Description
NOURIANZ contains istradefylline, an adenosine receptor antagonist, which has a xanthine derivative structure. The chemical name is (E)-8-(3,4-dimethoxystyryl)-1,3-diethyl-7-methyl-3,7-dihydro-1H-purine-2,6-dione. Its molecular formula is C20H24N4O4. The molecular weight is 384.43. Istradefylline has the following structural formula:
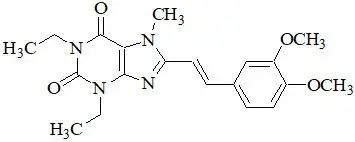
Istradefylline is a light yellow-green crystalline powder. Istradefylline has a dissociation constant (pKa) of 0.78. The aqueous solubility of istradefylline is ~0.5 µg/mL across the physiological pH range and 0.6 µg/mL in water.
NOURIANZ tablets are intended for oral administration only. Each tablet contains 20 mg or 40 mg of istradefylline and the following inactive ingredients: crospovidone, lactose monohydrate, magnesium stearate, microcrystalline cellulose, and polyvinyl alcohol. The film coating contains hypromellose, lactose monohydrate, polyethylene glycol 3350, titanium dioxide, triacetin, and the following dyes: iron oxide red and iron oxide yellow. Carnauba wax is used for polishing.
12. Nourianz - Clinical Pharmacology
12.1 Mechanism of Action
The precise mechanism by which istradefylline exerts its therapeutic effect in Parkinson disease is unknown. In in vitro studies and in in vivo animal studies, istradefylline was demonstrated to be an adenosine A2A receptor antagonist.
12.3 Pharmacokinetics
Istradefylline exhibits dose-proportional pharmacokinetics after multiple oral doses from 20 mg to 80 mg (2 times the maximum recommended dosage). Steady-state was reached within 2 weeks of once-daily dosing. The pharmacokinetics of istradefylline were similar in PD patients and healthy subjects.
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
Oral administration of istradefylline (0, 30, 100, or 320 mg/kg/day) to rats for two years resulted in an increase in the incidence and severity of vascular mineralization in the brain (including in the caudate/putamen, globus pallidus, thalamus, and nucleus accumbens) at all doses tested. The vascular mineralization was composed of calcium and phosphorus and, at higher doses, were reported to partially or completely occlude the blood vessels. There was no evidence of neuronal degeneration, inflammation, or glial response associated with the foci of mineralization.
Brain mineralization was not detected in mice administered istradefylline (0, 25, 125, or 250 mg/kg/day) orally for two years or in dogs administered istradefylline (0, 10, 30, or 100 mg/kg/day) orally for 52 weeks.
14. Clinical Studies
The efficacy of NOURIANZ for the adjunctive treatment to levodopa/carbidopa in patients with Parkinson's disease experiencing "off" episodes was shown in four randomized, multicenter, double-blind, 12-week, placebo-controlled studies (Study 1, NCT00456586; Study 2, NCT00199407; Study 3, NCT00455507; and Study 4, NCT00955526). The studies enrolled patients with a mean duration of Parkinson's disease of 9 years (range: 1 month to 37 years) that were Hoehn and Yahr Stage II to IV, experiencing at least 2 hours (mean approximately 6 hours) of "off" time per day, and were treated with levodopa for at least one year, with stable dosage for at least 4 weeks before screening (mean total daily dosage range: 416 to 785 mg). Patients continued levodopa treatment with or without concomitant PD medications, including dopamine agonists (85%), COMT inhibitors (38%), MAO-B inhibitors (40%), anticholinergics (13%), and/or amantadine (33%), provided the medications were stable for at least 4 weeks before screening and throughout the study period. The studies excluded patients who had received a neurosurgical treatment for PD (e.g., pallidotomy, thalamotomy, deep brain stimulation).
The primary efficacy endpoint was the change from baseline in the daily awake percentage of "off" time, or the change from baseline in total daily "off" time, based on 24-hour diaries completed by patients. A change from baseline in "on" time without troublesome dyskinesia (i.e., "on" time without dyskinesia plus "on" time with non-troublesome dyskinesia) was a secondary efficacy endpoint.
Study 1 was conducted in the U.S. and Canada, and Study 2 was conducted in the U.S. In these studies, patients were randomized to once-daily treatment with NOURIANZ 20 mg, 40 mg, or placebo. Patients treated with NOURIANZ 20 mg or NOURIANZ 40 mg once daily experienced a statistically significant decrease from baseline in percentage of daily awake "off" time, compared with patients on placebo, as summarized in Table 2.
| Baseline | Change from Baseline to Endpoint | |||
|---|---|---|---|---|
| N | (mean ± SD) % of awake "off" hours | N | (LSMD* vs. placebo), % awake "off" hours, (p-value) |
|
| SD: Standard Deviation | ||||
|
||||
| Study 1 | ||||
| Placebo | 66 | 37.2 ± 13.8 | 65 | -- |
| NOURIANZ 40 mg | 129 | 38.4 ± 16.2 | 126 | - 6.78 (p=0.007) |
| Study 2 | ||||
| Placebo | 113 | 38.7 ± 11.6 | 113 | -- |
| NOURIANZ 20 mg | 112 | 39.8 ± 14.0 | 112 | - 4.57 (p=0.025) |
Compared with patients on placebo, patients treated with NOURIANZ experienced an additional increase from baseline in "on" time without troublesome dyskinesia of 0.96 hours (nominal p=0.026) in Study 1, and of 0.55 hours (nominal p=0.135) in Study 2.
Study 3 and Study 4 were conducted in Japan. In these studies, patients were randomized equally to treatment with NOURIANZ 20 mg, 40 mg, or placebo. Patients treated with NOURIANZ 20 mg or NOURIANZ 40 mg once daily experienced a statistically significant decrease from baseline in "off" time compared with patients on placebo, as summarized in Table 3.
| Baseline | Change from Baseline to Endpoint | |||
|---|---|---|---|---|
| N | (mean ± SD) hours | N | (LSMD* vs. placebo) hours ( p-value) |
|
| SD: Standard Deviation | ||||
|
||||
| Study 3 | ||||
| Placebo | 118 | 6.4 ± 2.7 | 118 | -- |
| NOURIANZ 20 mg | 115 | 6.8 ± 2.9 | 115 | -0.65 (p=0.028) |
| NOURIANZ 40 mg | 124 | 6.6 ± 2.5 | 124 | -0.92 (p=0.002) |
| Study 4 | ||||
| Placebo | 123 | 6.3 ± 2.5 | 123 | -- |
| NOURIANZ 20 mg | 120 | 6.6 ± 2.7 | 120 | -0.76 (p=0.006) |
| NOURIANZ 40 mg | 123 | 6.0 ± 2.5 | 123 | -0.74 (p=0.008) |
In Study 3, compared with placebo, an additional increase from baseline in "on" time without troublesome dyskinesia of 0.57 hours (nominal p=0.085) and of 0.65 hours (nominal p=0.048), respectively, were observed in patients treated with NOURIANZ 20 mg or NOURIANZ 40 mg. In Study 4, the corresponding increases in "on" time without troublesome dyskinesia were 0.83 hours (nominal p=0.008) for NOURIANZ 20 mg and 0.81 hours (nominal p=0.008) for NOURIANZ 40 mg.
16. How is Nourianz supplied
16.1 How Supplied
NOURIANZ (istradefylline) tablets are available as:
20 mg Tablets:
Peach-colored, pillow-shaped, film-coated tablets with "20" debossed on one side.
Bottle of 90: NDC 42747-602-90
40 mg Tablets:
Peach-colored, almond-shaped, film-coated tablets with "40" debossed on one side.
Bottle of 90: NDC 42747-604-90
| NOURIANZ
istradefylline tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| NOURIANZ
istradefylline tablet, film coated |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Kyowa Kirin, Inc. (014778321) |