Drug Detail:Nplate (Romiplostim [ rom-i-plos-tim ])
Drug Class: Platelet-stimulating agents
Highlights of Prescribing Information
NPLATE® (romiplostim) for injection, for subcutaneous use
Initial U.S. Approval: 2008
Recent Major Changes
| Dosage and Administration (2.3) | 02/2022 |
Indications and Usage for Nplate
Nplate is a thrombopoietin receptor agonist indicated for the treatment of thrombocytopenia in:
- Adult patients with immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. (1.1)
- Pediatric patients 1 year of age and older with ITP for at least 6 months who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy. (1.1)
Nplate is indicated to increase survival in adults and in pediatric patients (including term neonates) acutely exposed to myelosuppressive doses of radiation (Hematopoietic Syndrome of Acute Radiation Syndrome [HS-ARS]). (1.2)
Limitations of Use:
- Nplate is not indicated for the treatment of thrombocytopenia due to myelodysplastic syndrome (MDS) or any cause of thrombocytopenia other than ITP.
- Nplate should be used only in patients with ITP whose degree of thrombocytopenia and clinical condition increases the risk for bleeding.
- Nplate should not be used in an attempt to normalize platelet counts. (1)
Nplate Dosage and Administration
- Patients with Immune Thrombocytopenia (ITP)
- Recommended Initial Dose: 1 mcg/kg once weekly as a subcutaneous injection. Adjust dose based on platelet response. (2.1)
- Patients acutely exposed to myelosuppressive doses of radiation
- Recommended Dose: 10 mcg/kg administered once as a subcutaneous injection. Administer the dose as soon as possible after suspected or confirmed exposure to myelosuppressive doses of radiation. (2.2)
- See Full Prescribing Information for instructions on reconstitution, preparation, and administration. (2.3)
Dosage Forms and Strengths
- For injection: 125 mcg, 250 mcg or 500 mcg of romiplostim as a lyophilized powder in single-dose vials. (3)
Contraindications
None (4)
Warnings and Precautions
- In some patients with MDS, Nplate increases blast cell counts and increases the risk of progression to acute myelogenous leukemia. (5.1)
- Thrombotic/thromboembolic complications may result from increases in platelet counts with Nplate use. Portal vein thrombosis has been reported in patients with chronic liver disease receiving Nplate. (5.2)
- If severe thrombocytopenia develops during Nplate treatment, assess patients for the formation of neutralizing antibodies. (5.3)
Adverse Reactions/Side Effects
- In adult patients, the most common adverse reactions (≥ 5% higher patient incidence in Nplate versus placebo) are arthralgia, dizziness, insomnia, myalgia, pain in extremity, abdominal pain, shoulder pain, dyspepsia, and paresthesia. Headache was the most commonly reported adverse reaction that did not occur at ≥ 5% higher patient incidence in Nplate versus placebo. (6.1)
- In pediatric patients, the most common adverse reactions (≥ 25%) are: contusion, upper respiratory tract infection, and oropharyngeal pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Amgen Inc. at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Pregnancy: May cause fetal harm. (8.1)
- Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 2/2022
Related/similar drugs
prednisone, dexamethasone, triamcinolone, Decadron, PromactaFull Prescribing Information
1. Indications and Usage for Nplate
1.1 Patients with Immune Thrombocytopenia (ITP)
Nplate is indicated for the treatment of thrombocytopenia in:
- Adult patients with immune thrombocytopenia (ITP) who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
- Pediatric patients 1 year of age and older with ITP for at least 6 months who have had an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
1.2 Patients with Hematopoietic Syndrome of Acute Radiation Syndrome
Nplate is indicated to increase survival in adults and in pediatric patients (including term neonates) acutely exposed to myelosuppressive doses of radiation [see Clinical Studies (14.3)].
Limitations of Use:
- Nplate is not indicated for the treatment of thrombocytopenia due to myelodysplastic syndrome (MDS) or any cause of thrombocytopenia other than ITP [see Warnings and Precautions (5.1)].
- Nplate should be used only in patients with ITP whose degree of thrombocytopenia and clinical condition increases the risk for bleeding.
- Nplate should not be used in an attempt to normalize platelet counts [see Warnings and Precautions (5.2)].
2. Nplate Dosage and Administration
2.1 Patients with Immune Thrombocytopenia (ITP)
Use the lowest dose of Nplate to achieve and maintain a platelet count ≥ 50 × 109/L as necessary to reduce the risk for bleeding. Administer Nplate as a weekly subcutaneous injection with dose adjustments based upon the platelet count response.
The prescribed Nplate dose may consist of a very small volume (e.g., 0.15 mL). Administer Nplate only with a syringe that contains 0.01 mL graduations.
Discontinue Nplate if the platelet count does not increase to a level sufficient to avoid clinically important bleeding after 4 weeks of Nplate therapy at the maximum weekly dose of 10 mcg/kg [see Warnings and Precautions (5.3)].
Obtain complete blood counts (CBCs), including platelet counts, weekly during the dose adjustment phase of Nplate therapy and then monthly following establishment of a stable Nplate dose. Obtain CBCs, including platelet counts, weekly for at least 2 weeks following discontinuation of Nplate.
2.3 Preparation and Administration
To mitigate against medication errors (both overdose and underdose), ensure that these preparation and administration instructions are followed. Use aseptic technique. Only administer subcutaneously [see Overdosage (10)].
Nplate is supplied in single-dose vials as a sterile, preservative-free, white lyophilized powder that must be reconstituted as outlined in Table 1 and administered using a syringe with 0.01 mL graduations.
Calculation of Patient Dose
Multiply the patient’s weight (kg) by the prescribed dose to obtain the Calculated Patient Dose.
| Calculated Patient Dose (mcg) = Weight (kg) × Prescribed dose (mcg/kg) |
Reconstitution and Dilution of Nplate Single-Dose Vials
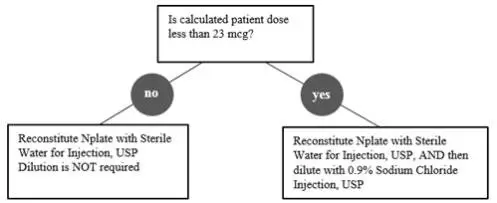
Reconstitute Nplate with Sterile Water for Injection, USP. Do not reconstitute or dilute with Bacteriostatic Water for Injection, USP or dilute with Bacteriostatic Sodium Chloride Injection, USP. If the Calculated Patient Dose is less than 23 mcg, dilution with 0.9% Sodium Chloride Injection, USP is required to reduce the concentration of Nplate (see Table 1).This reduced concentration allows for low-doses to be accurately calculated, and consistently measured with a 0.01 mL graduated syringe.
| Calculated Patient Dose | Strength* | Reconstitute with Sterile Water† | Dilute with Normal Saline‡ | Final Concentration |
|---|---|---|---|---|
|
||||
| Calculated Dose greater than or equal to 23 mcg | 125 mcg | 0.44 mL | Not Required | 500 mcg/mL |
| 250 mcg | 0.72 mL | Not Required | ||
| 500 mcg | 1.2 mL | Not Required | ||
| Calculated Dose less than 23 mcg | 125 mcg | 0.44 mL | 1.38 mL | 125 mcg/mL |
| 250 mcg | 0.72 mL | 2.25 mL | ||
| 500 mcg | 1.2 mL | 3.75 mL | ||
Gently swirl and invert the vial to reconstitute. Avoid excess or vigorous agitation: DO NOT SHAKE. Generally, dissolution of Nplate takes less than 2 minutes. The reconstituted Nplate solution should be clear and colorless. Visually inspect the reconstituted solution for particulate matter and/or discoloration. Do not administer Nplate if particulate matter and/or discoloration is observed.
Calculate Volume to Administer by dividing the Calculated Patient Dose (mcg) by the final concentration of prepared solution. See Table 2 for final concentrations.
| Calculated Patient Dose | Final Concentration | Volume to Administer (mL) |
| Calculated Dose greater than or equal to 23 mcg | 500 mcg/mL | = Calculated Patient Dose / 500 mcg/mL |
| Calculated Dose less than 23 mcg | 125 mcg/mL | = Calculated Patient Dose / 125 mcg/mL |
Administration of Prepared Nplate Solution
Administer Nplate only using a syringe with 0.01 mL graduations for accurate dosage. Round volume to the nearest hundredth mL. Verify that the syringe contains the correct dosage.
Discard any unused portion. Do not pool unused portions from the vials. Do not administer more than one dose from a vial.
Storage of Reconstituted Solution
Reconstituted product with Sterile Water for Injection, USP that has not been further diluted can remain in the original vial at room temperature 25°C (77°F) or be refrigerated at 2°C to 8°C (36°F to 46°F) for up to 24 hours following reconstitution. Reconstituted product with Sterile Water for Injection, USP may be held in a syringe at room temperature 25°C (77°F) for a maximum of 4 hours following reconstitution. Protect product from light. Do not shake.
Storage of Diluted solution (after initial reconstitution)
Reconstituted and further diluted product with 0.9% Sodium Chloride Injection, USP can be held in a syringe at room temperature 25°C (77°F) or in the original vial refrigerated at 2°C to 8°C (36°F to 46°F) for no longer than 4 hours prior to administration. Protect product from light. Do not shake.
3. Dosage Forms and Strengths
For injection: 125 mcg, 250 mcg or 500 mcg of Nplate as a sterile, lyophilized, solid white powder in single-dose vials.
5. Warnings and Precautions
5.1 Risk of Progression of Myelodysplastic Syndromes to Acute Myelogenous Leukemia
Progression from myelodysplastic syndromes (MDS) to acute myelogenous leukemia (AML) has been observed in adult clinical trials with Nplate.
A randomized, double-blind, placebo-controlled trial enrolling adult patients with severe thrombocytopenia and International Prognostic Scoring System (IPSS) low or intermediate-1 risk MDS was terminated due to more cases of AML observed in the Nplate arm. This trial consisted of a 58-week study period with a 5-year long-term follow-up phase. The patients were randomized 2:1 to treatment with Nplate or placebo (167 Nplate, 83 placebo). During the 58-week study period, progression to AML occurred in 10 (6.0%) patients in the Nplate arm and 4 (4.8%) patients in the placebo arm (hazard ratio [95%CI] = 1.20 [0.38, 3.84]). Of the 250 patients, 210 (84.0%) entered the long-term follow-up phase of this study. With 5-years of follow-up, 29 (11.6%) patients showed progression to AML, including 20/168 (11.9%) patients in the Nplate arm versus 9/82 (11.0%) patients in the placebo arm (HR [95% CI] = 1.06 [0.48, 2.33]). The incidence of death (overall survival) was 55.7% (93/167) in the Nplate arm versus 54.2% (45/83) in the placebo arm (HR [95% CI] = 1.03 [0.72, 1.47]). In the baseline low IPSS group, there was a higher incidence of death in the Nplate arm [41.3% (19/46)] compared to the placebo arm [30.4% (7/23)] (HR [95% CI] = 1.59 [0.67, 3.80]).
In a single-arm trial of Nplate given to 72 patients with thrombocytopenia-related MDS, 8 (11.1%) patients were reported as having possible disease progression, of which 3 (4.2%) had confirmation of AML during follow-up. In addition, in 3 (4.2%) patients, increased peripheral blood blast cell counts decreased to baseline after discontinuation of Nplate.
Nplate is not indicated for the treatment of thrombocytopenia due to MDS or any cause of thrombocytopenia other than ITP.
5.2 Thrombotic/Thromboembolic Complications
Thrombotic/thromboembolic complications may result from increases in platelet counts with Nplate use secondary to drug-induced thrombocytosis, regardless of the underlying disease. There is insufficient evidence to establish a relationship between maximum platelet threshold and risk of thrombotic/thromboembolic complications. Portal vein thrombosis has been reported in patients with chronic liver disease receiving Nplate.
In patients with ITP, to minimize the risk for thrombotic/thromboembolic complications, do not use Nplate in an attempt to normalize platelet counts. Follow the dose adjustment guidelines [see Dosage and Administration (2.1)].
In the absence of myelosuppression induced by acute exposure to radiation, Nplate administration might cause excessive increases in platelet counts and may cause thrombotic and thromboembolic complications [see Clinical Pharmacology (12.2)].
5.3 Loss of Response to Nplate
Hyporesponsiveness or failure to maintain a platelet response with Nplate should prompt a search for causative factors, including neutralizing antibodies to Nplate [see Adverse Reactions (6.3)]. To detect antibody formation, submit blood samples to Amgen (1-800-772-6436). Amgen will assay these samples for antibodies to Nplate and thrombopoietin (TPO). Discontinue Nplate if the platelet count does not increase to a level sufficient to avoid clinically important bleeding after 4 weeks at the highest weekly dose of 10 mcg/kg.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are discussed in greater detail in other sections:
- Progression of Myelodysplastic Syndromes [see Warnings and Precautions (5.1)]
- Thrombotic/Thromboembolic Complications [see Warnings and Precautions (5.2)]
- Loss of Response to Nplate [see Warnings and Precautions (5.3)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adults
The data described below reflect Nplate exposure to 271 adult patients with ITP, aged 18 to 88, of whom 62% were female. Nplate was studied in two randomized, placebo-controlled, double-blind studies that were identical in design, with the exception that Study 1 evaluated nonsplenectomized patients with ITP and Study 2 evaluated splenectomized patients with ITP. Data are also reported from an open-label, single-arm study in which patients received Nplate over an extended period of time. Overall, Nplate was administered to 114 patients for at least 52 weeks and 53 patients for at least 96 weeks.
In the placebo-controlled studies, headache was the most commonly reported adverse drug reaction, occurring in 35% of patients receiving Nplate and 32% of patients receiving placebo. For those patients receiving Nplate, 14 (48%) of headaches were mild, 9 (31%) were moderate, and 6 (21%) were severe. Table 3 presents adverse drug reactions from Studies 1 and 2 with a ≥ 5% higher patient incidence in Nplate versus placebo.
| Adverse Reactions by Body System | Nplate (%)
(n=84) | Placebo (%)
(n=41) |
| Musculoskeletal and Connective Tissue Disorders | ||
| Arthralgia | 22 (26%) | 8 (20%) |
| Myalgia | 12 (14%) | 1 (2%) |
| Pain in Extremity | 11 (13%) | 2 (5%) |
| Shoulder Pain | 7 (8%) | 0 |
| Nervous System Disorders | ||
| Dizziness | 14 (17%) | 0 |
| Paresthesia | 5 (6%) | 0 |
| Psychiatric Disorders | ||
| Insomnia | 13 (16%) | 3 (7%) |
| Gastrointestinal Disorders | ||
| Abdominal pain | 9 (11%) | 0 |
| Dyspepsia | 6 (7%) | 0 |
| MedDRA version 9 is used. | ||
Among 291 adult patients with ITP who received Nplate in the single-arm extension study, the incidence rates of the adverse reactions occurred in a pattern similar to those reported in the placebo-controlled clinical studies.
The safety profile of Nplate was similar across patients, regardless of ITP duration. The following adverse reactions (at least 5% incidence and at least 5% more frequent with Nplate compared with placebo or standard of care) occurred in Nplate patients with ITP duration up to 12 months: bronchitis, sinusitis, vomiting, arthralgia, myalgia, headache, dizziness, diarrhea, upper respiratory tract infection, cough, nausea and oropharyngeal pain. The adverse reaction of thrombocytosis occurred with an incidence of 2% in adults with ITP duration up to 12 months.
Bone Marrow Reticulin Formation and Collagen Fibrosis
Nplate administration may increase the risk for development or progression of reticulin fiber formation within the bone marrow. This formation may improve upon discontinuation of Nplate. In a clinical trial, one patient with ITP and hemolytic anemia developed marrow fibrosis with collagen during Nplate therapy. An open-label clinical trial prospectively evaluated changes in bone marrow reticulin formation and collagen fibrosis in adult patients with ITP treated with Nplate or a non-US approved romiplostim product. Patients were administered romiplostim by SC injection once weekly for up to 3 years. Based on cohort assignment at time of study enrollment, patients were evaluated for bone marrow reticulin and collagen at year 1 (cohort 1), year 2 (cohort 2), or year 3 (cohort 3) in comparison to the baseline bone marrow at start of the trial. Patients were evaluated for bone marrow reticulin formation and collagen fibrosis using the modified Bauermeister grading scale. From the total of 169 patients enrolled in the 3 cohorts, 132 (78%) patients were evaluable for bone marrow collagen fibrosis and 131 (78%) patients were evaluable for bone marrow reticulin formation. Two percent (2/132) of patients (both from cohort 3) developed Grade 4 findings (presence of collagen). There was no detectable bone marrow collagen in one patient on repeat testing 12 weeks after discontinuation of romiplostim. Progression of bone marrow reticulin formation (increase greater than or equal to 2 grades or more) or an increase to Grade 4 (presence of collagen) was reported in 7% (9/131) of patients.
Pediatric Patients
The data described below reflect median exposure to Nplate of 168 days for 59 pediatric patients (aged 1 to 17 years) with ITP for at least 6 months, of whom 47.5% were female, across the randomized phase of two placebo-controlled trials. Table 4 presents the most common adverse reactions experienced by at least 5% of the pediatric patients (1 year and older) receiving Nplate across the two placebo-controlled trials with at least a 5% higher incidence in patients who received Nplate compared to those who received placebo.
| MedDRA version 20.1 is used. In pediatric patients of age ≥ 1 year receiving Nplate for ITP, adverse reactions with an incidence of ≥ 25% in the two randomized trials were: contusion (41%), upper respiratory tract infection (31%), and oropharyngeal pain (25%). |
||
| Adverse Reactions by Body System | Nplate (%)
(N = 59) | Placebo (%)
(N = 24) |
| Infections and Infestations | ||
| Upper Respiratory Tract Infection | 18 (31%) | 6 (25%) |
| Ear Infection | 3 (5%) | 0 |
| Gastroenteritis | 3 (5%) | 0 |
| Sinusitis | 3 (5%) | 0 |
| Respiratory, Thoracic and Mediastinal Disorders | ||
| Oropharyngeal Pain | 15 (25%) | 1 (4%) |
| Gastrointestinal Disorders | ||
| Diarrhea | 12 (20%) | 3 (13%) |
| Abdominal Pain Upper | 8 (14%) | 1 (4%) |
| Skin and Subcutaneous Tissue Disorders | ||
| Rash | 9 (15%) | 2 (8%) |
| Purpura | 4 (7%) | 0 |
| Urticaria | 3 (5%) | 0 |
| General Disorders and Administration Site Conditions | ||
| Pyrexia | 14 (24%) | 2 (8%) |
| Peripheral Swelling | 4 (7%) | 0 |
| Injury, Poisoning and Procedural Complications | ||
| Contusion | 24 (41%) | 8 (33%) |
Among 203 pediatric patients with ITP who received Nplate in a single arm, open-label, long-term (median duration of 3 years on therapy) study, the incidence rates of the adverse reactions occurred in a pattern similar to those reported in the placebo-controlled clinical studies. In this single arm, open-label, long-term study, headache occurred in 78 patients (38%), 3% (n=6) being severe and 1% (n=2) resulting in discontinuation of drug.
6.3 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to Nplate in the studies described below with the incidence of antibodies in other studies or to other products may be misleading. Patients were screened for immunogenicity to romiplostim using a BIAcore-based biosensor immunoassay. This assay is capable of detecting both high- and low-affinity binding antibodies that bind to romiplostim and cross-react with TPO. The samples from patients that tested positive for binding antibodies were further evaluated for neutralizing capacity using a cell-based bioassay.
In adult clinical studies in adult patients with ITP, the incidence of pre-existing antibodies to romiplostim was 3.3% (35/1046) and the incidence of binding antibody development during treatment with Nplate or a non-US approved romiplostim product was 5.7% (60/1046). The incidence of pre-existing antibodies to endogenous TPO was 3% (31/1046) and the incidence of binding antibody development to endogenous TPO during treatment was 3.2% (33/1046). Of the patients with positive binding antibodies that developed to romiplostim or to TPO, four patients had neutralizing activity to romiplostim and none had neutralizing activity to TPO. No apparent correlation was observed between antibody activity and clinical effectiveness or safety.
In pediatric studies, data on antibody formation was collected from 282 patients (20 from early phase studies, 59 from phase 3 studies with duration of 6 months and 203 from a long-term study with median duration of 3 years). The incidence of binding antibodies to Nplate at any time was 9.6% (27/282), of which 2 patients (0.7%) had pre-existing binding non-neutralizing Nplate antibodies at baseline and 11 patients (3.9%) had persistent binding antibody positivity at end of study. Additionally, 2.8% (8/282) developed neutralizing antibodies to Nplate, with 4 patients (1.4%) having persistent neutralizing antibody positivity at end of study, despite discontinuation of Nplate. The incidence of binding antibodies to TPO at any time was 3.9% (11/282), of which 2 patients (0.7%) had pre-existing binding non-neutralizing antibodies to TPO at baseline and 1 patient (0.4%) had binding persistent antibody positivity at end of study. One patient (0.4%) had a weakly positive postbaseline result for neutralizing antibodies against TPO while on study (with positive non-neutralizing antibodies to Nplate) with a negative result at baseline for both antibodies. The patient showed a transient antibody response for neutralizing antibodies against TPO, with a negative result at the patient's last timepoint tested within the study period after discontinuation of Nplate.
A postmarketing registry study involving patients with thrombocytopenia on Nplate or a non-US approved romiplostim product was conducted to assess the long-term consequences of the anti-romiplostim antibodies. Adult patients who lacked response or lost response to Nplate or a non-US approved romiplostim product were enrolled. The incidence of new binding antibody development was 3.8% (7/184) to romiplostim and 2.2% (4/184) were positive for binding, non-neutralizing antibodies to TPO; two patients were positive for binding antibodies to both romiplostim and TPO. Of the seven patients with positive binding antibodies to romiplostim, one patient (0.5%; 1/184) was positive for neutralizing antibodies to romiplostim only.
Nineteen confirmed pediatric patients were included in the postmarketing registry study. The incidence of binding antibody post treatment was 16% (3/19) to romiplostim, of which 5.3% (1/19) were positive for neutralizing antibodies to romiplostim. There were no antibodies detected to TPO.
Immunogenicity assay results are highly dependent on the sensitivity and specificity of the assay used in detection and may be influenced by several factors, including sample handling, concomitant medications, and underlying disease. For these reasons, comparison of incidence of antibodies to romiplostim with the incidence of antibodies to other products may be misleading.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Based on findings from animal reproduction studies, Nplate may cause fetal harm when administered to a pregnant woman. Available data with Nplate use in pregnant women are insufficient to draw conclusions about any drug-associated risk for major birth defects, miscarriage, or adverse maternal or fetal outcomes. In animal reproduction and developmental toxicity studies, romiplostim crossed the placenta, and adverse fetal effects included thrombocytosis, postimplantation loss, and an increase in pup mortality (see Data).
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risks of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
Data
Animal Data
In rat and rabbit embryo-fetal development toxicity studies, no evidence of fetal harm was observed at romiplostim doses up to 11 times (rats) and 82 times (rabbits) the maximum human dose (MHD) based on systemic exposure (AUC). In mice at doses 5 times the MHD, reductions in maternal body weight and increased postimplantation loss occurred.
In a prenatal and postnatal development study in rats, at doses 11 times the MHD, there was an increase in perinatal pup mortality. Romiplostim crossed the placental barrier in rats and increased fetal platelet counts at clinically equivalent and higher doses.
8.2 Lactation
Risk Summary
There is no information regarding the presence of romiplostim in human milk, the effects on the breastfed child, or the effects on milk production. Maternal IgG is known to be present in human milk. The effects of local gastrointestinal exposure and limited systemic exposure in the breastfed child to romiplostim are unknown. Due to the potential for serious adverse reactions in a breastfed child from Nplate, advise women not to breastfeed during treatment with Nplate.
10. Overdosage
Overdoses due to medication errors have been reported in patients receiving Nplate. In the event of overdose, platelet counts may increase excessively and result in thrombotic/thromboembolic complications. In this case, discontinue Nplate and monitor platelet counts. Reinitiate treatment with Nplate in accordance with dosing and administration recommendations [see Dosage and Administration (2.1, 2.3)].
12. Nplate - Clinical Pharmacology
12.2 Pharmacodynamics
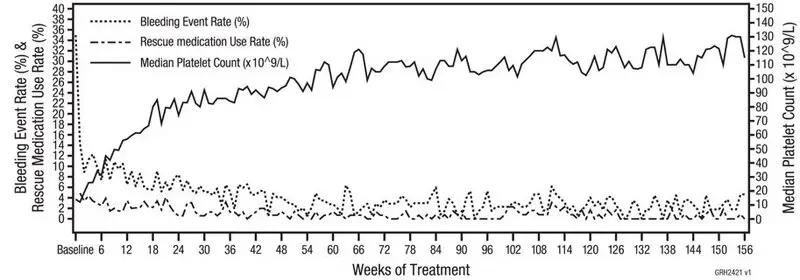
In clinical studies, treatment with Nplate resulted in dose-dependent increases in platelet counts. After a single subcutaneous dose of 1 to 10 mcg/kg Nplate in patients with ITP, the peak platelet count was 1.3 to 14.9 times greater than the baseline platelet count over a 2- to 3-week period. The platelet counts were above 50 × 109/L for seven out of eight patients with ITP who received six weekly doses of Nplate at 1 mcg/kg.
In a clinical study, peak platelet count increased 4.7 to 7.3 fold (mean: 5.8 fold) above baseline values in healthy adults (n = 4) administered a single 10 mcg/kg IV dose of Nplate.
Results from population modeling and simulation indicate that a single 10 mcg/kg subcutaneous dose of Nplate would result in clinically relevant effects on incidence rate and duration of severe thrombocytopenia in patients acutely exposed to myelosuppressive doses of radiation.
14. Clinical Studies
14.2 Pediatric Patients with ITP
The safety and efficacy of Nplate in pediatric patients 1 year and older with ITP for at least 6 months were assessed in two double-blind, placebo-controlled clinical trials.
Study 5 (NCT01444417)
In Study 5, patients refractory or relapsed after at least one prior ITP therapy with a platelet count ≤ 30 x 109/L were stratified by age and randomized (2:1) to receive Nplate (n = 42) or placebo (n = 20). The starting dose for all ages was 1 mcg/kg weekly. Over a 24-week treatment period, dose was titrated up to a maximum of 10 mcg/kg weekly of either Nplate or placebo in an effort to maintain a target platelet count of ≥ 50 × 109/L to 200 × 109/L.
The median age of the patients was 9.5 years (range 3 to 17) and 57% were female. Approximately 58% of patients had a baseline count ≤ 20 x 109/L, which was similar between treatment arms. The percentage of patients with at least 2 prior ITP therapies (predominantly immunoglobulins and corticosteroids) was 81% in the group treated with Nplate and 70% in the group treated with placebo. One patient in each group had undergone splenectomy.
Study 5 results are shown in Table 6. The efficacy of Nplate in this trial was measured by the proportion of patients receiving Nplate achieving a durable platelet response and the proportion of patient achieving an overall platelet response. A durable platelet response was defined as achieving at least 6 weekly platelet counts ≥ 50 × 109/L during weeks 18 through 25 of treatment. A transient platelet response was defined as a weekly platelet count ≥ 50 × 109/L for 4 or more times during weeks 2 through 25, but without durable platelet response. An overall platelet response was defined as a durable or a transient platelet response. Platelet responses were excluded for 4 weeks after receiving rescue medications.
| Outcomes | Study 5 | |
| Nplate
(n = 42) | Placebo
(n = 20) |
|
| Platelet Responses and Rescue Therapy | ||
| Durable Platelet Responsea, n (%) | 22 (52%) | 2 (10%) |
| Overall Platelet Responsea, n (%) | 30 (71%) | 4 (20%) |
| Number of Weeks with Platelet Counts ≥ 50 x 109/L, mediana | 12 | 1 |
| a All p values < 0.05 for platelet response between Nplate and placebo. | ||
Study 6 (NCT00515203)
In study 6, patients diagnosed with ITP at least 6 months prior to enrollment with a platelet count ≤ 30 x 109/L were stratified by age and randomized (3:1) to receive Nplate (n = 17) or placebo (n = 5). The starting dose for all ages was 1 mcg/kg weekly. Over a 12-week treatment period dose was titrated up to a maximum of 10 mcg/kg weekly of either Nplate or placebo in an effort to maintain a target platelet count of ≥ 50 × 109/L to 250 × 109/L.
The median age of the patients was 10 years (range 1 to 17 years) and 27.3% of patients were female. Approximately 82% of patients had a baseline count ≤ 20 x 109/L, which was similar between treatment arms. The percentage of patients with at least 2 prior ITP therapies (predominantly IVIG and corticosteroids) was 88% in the group treated with Nplate and 100% in the group treated with placebo. Six patients in the Nplate group and 2 patients in the placebo group had undergone splenectomy.
The efficacy of Nplate in this trial was measured by the proportion of patients who achieved a platelet count of ≥ 50 × 109/L for 2 consecutive weeks and by the proportion of patients who achieved an increase in platelet count of ≥ 20 × 109/L above baseline for 2 consecutive weeks. Platelet responses within 4 weeks following rescue medications use were excluded. Of the 17 patients who received romiplostim, 15 achieved a platelet count of ≥ 50 × 109/L for 2 consecutive weeks (88.2%, 95% CI: 63.6%, 98.5%).
The same 15 patients also achieved an increase in platelet count of ≥ 20 × 109/L above baseline for 2 consecutive weeks during the treatment period (88.2%, 95% CI: 63.6%, 98.5%). None of the patients treated with placebo achieved either endpoint.
14.3 Patients with Hematopoietic Syndrome of Acute Radiation Syndrome
Efficacy studies of Nplate could not be conducted in humans with acute radiation syndrome for ethical and feasibility reasons. Approval for this indication was based on efficacy studies conducted in animals, Nplate's effect on platelet count in healthy human volunteers and on data supporting Nplate's effect on thrombocytopenia in patients with ITP and insufficient response to corticosteroids, immunoglobulins, or splenectomy.
Because of the uncertainty associated with extrapolating animal efficacy data to humans, the selection of a human dose for Nplate is aimed at providing platelet response to Nplate that is similar to that observed in efficacy studies conducted in animals. The recommended dose of Nplate for patients exposed to myelosuppressive doses of radiation is 10 mcg/kg administered once as a subcutaneous injection [see Dosage and Administration (2.2)]. The 10 mcg/kg dosing regimen for humans is based on population modeling and simulation analyses. For pediatric patients (including term neonates), extrapolation was based on data supporting Nplate's effect on thrombocytopenia in patients with ITP and an insufficient response to corticosteroids, immunoglobulins, or splenectomy.
The safety of Nplate for the acute radiation syndrome setting was assessed based on the clinical experience in patients with ITP [see Adverse Reactions (6)] and from a study with healthy volunteers. The efficacy of Nplate was studied in a randomized, blinded, placebo-controlled study in a non-human primate model of radiation injury. Rhesus monkeys were randomized to either a control (n = 40) or treated (n = 40) cohort. Animals were exposed to total body irradiation (TBI) of 6.8 Gy from a Cobalt60 gamma ray source, representing a dose that would be lethal in 70% of animals by 60 days of follow-up (LD70/60). Animals were administered a single subcutaneous dose of blinded treatment (control article [sterile saline] or Nplate [5mg/kg]) 24 hours post-irradiation. The primary efficacy endpoint was survival. Animals received medical management consisting of intravenous or subcutaneous fluids, anti-ulcer medication, anti-emetic medication, analgesics, antimicrobials, and other support as required.
Nplate significantly (one-sided p = 0.0002) increased 60-day survival in the irradiated animals: 72.5% survival (29/40) in the Nplate group compared to 32.5% survival (13/40) in the control group. In the same study, an exploratory cohort of n=40 animals received Nplate (5mg/kg) on day 1 and pegfilgrastim (0.3mg/kg) on days 1 and 8 post-irradiation. Survival in this combined treatment group was 87.5% (95% CI: (73.2%, 95.8%)).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: 01/2021 | |
| MEDICATION GUIDE
Nplate® (N-plāt) (romiplostim) for injection |
|
| What is the most important information I should know about Nplate?
Nplate can cause serious side effects, including:
|
|
What is Nplate?
|
|
Before receiving Nplate, first speak to your healthcare provider and understand the benefits and risks of Nplate. Be sure to tell your healthcare provider about all of your medical conditions, including if you:
Know the medicines you take. Keep a list of them and show it to your healthcare provider or pharmacist when you get a new medicine. |
|
How will I receive Nplate?
|
|
| What should I avoid while receiving Nplate?
Avoid situations or medicines that may increase your risk of bleeding. |
|
| What are the possible side effects of Nplate?
Nplate may cause serious side effects. See “What is the most important information I should know about Nplate?” The most common side effects of Nplate in adults include: |
|
| • headache | • tingling or numbness in hands and feet |
| • joint pain | • bronchitis |
| • dizziness | • inflammation of the sinuses (sinusitis) |
| • trouble sleeping | • vomiting |
| • muscle tenderness or weakness • pain in the arms and legs • stomach (abdomen) pain • shoulder pain • indigestion | • diarrhea • upper respiratory tract infection • cough • nausea • pain in mouth and throat (oropharyngeal pain) |
| The most common side effects of Nplate in children 1 year of age and older include: | |
| • bruising • upper respiratory tract infection | • pain in mouth and throat (oropharyngeal pain) |
| People who take Nplate may have an increased risk of developing new or worsening changes in the bone marrow called “increased reticulin”. These changes may improve if you stop taking Nplate. Your healthcare provider may need to check your bone marrow for this problem during treatment with Nplate. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. You may also report side effects to Amgen at 1-800-77-AMGEN (1-800-772-6436). |
|
| General information about the safe and effective use of Nplate.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about Nplate that is written for health professionals. |
|
| What are the ingredients in Nplate?
Active ingredient: romiplostim Inactive ingredients: L-histidine, mannitol, polysorbate 20, sucrose, and hydrochloric acid Nplate® (romiplostim) Manufactured by: Amgen Inc., One Amgen Center Drive, Thousand Oaks, California 91320-1799 US License No. 1080. Patent: http://pat.amgen.com/nplate/ © 2008-2021 Amgen Inc. All rights reserved. For more information go to www.nplate.com. 1xxxxxx v9 |
|
| NPLATE
romiplostim injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NPLATE
romiplostim injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| NPLATE
romiplostim injection, powder, lyophilized, for solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amgen Inc (039976196) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amgen Manufacturing Ltd | 785800020 | ANALYSIS(55513-221, 55513-222, 55513-223) , API MANUFACTURE(55513-221, 55513-222, 55513-223) , PACK(55513-221, 55513-222, 55513-223) , LABEL(55513-221, 55513-222, 55513-223) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amgen, Inc | 039976196 | ANALYSIS(55513-221, 55513-222, 55513-223) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Immunex Rhode Island Corporation | 968084785 | ANALYSIS(55513-221, 55513-222, 55513-223) | |