Drug Detail:Nymalize (Nimodipine [ nih-mo-dih-peen ])
Drug Class: Calcium channel blocking agents
Highlights of Prescribing Information
NYMALIZE (nimodipine) oral solution
Initial U.S. Approval: 1988
Indications and Usage for Nymalize
NYMALIZE is a dihydropyridine calcium channel blocker indicated for the improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in adult patients with subarachnoid hemorrhage (SAH) from ruptured intracranial berry aneurysms regardless of their post-ictus neurological condition (i.e., Hunt and Hess Grades I-V). (1)
Nymalize Dosage and Administration
- Administer only enterally (e.g., oral, nasogastric tube, or gastric tube route). Do not administer intravenously or by other parenteral routes. (2.1)
- Give one hour before a meal or two hours after a meal. (2.1)
- Start dosing within 96 hours of the SAH. (2.1)
- Recommended dose is 10 mL (60 mg) every 4 hours for 21 consecutive days. (2.2)
- Nasogastric or Gastric Tube Administration: Administer 10 mL (60 mg) every 4 hours with supplied prefilled oral syringe. Refill syringe with 10 mL of 0.9% saline water solution; flush remaining contents from nasogastric or gastric tube into stomach. (2.3)
- Patients with Cirrhosis: Reduce dosage to 5 mL (30 mg) every 4 hours. (2.4)
Dosage Forms and Strengths
Oral solution (6 mg per mL):
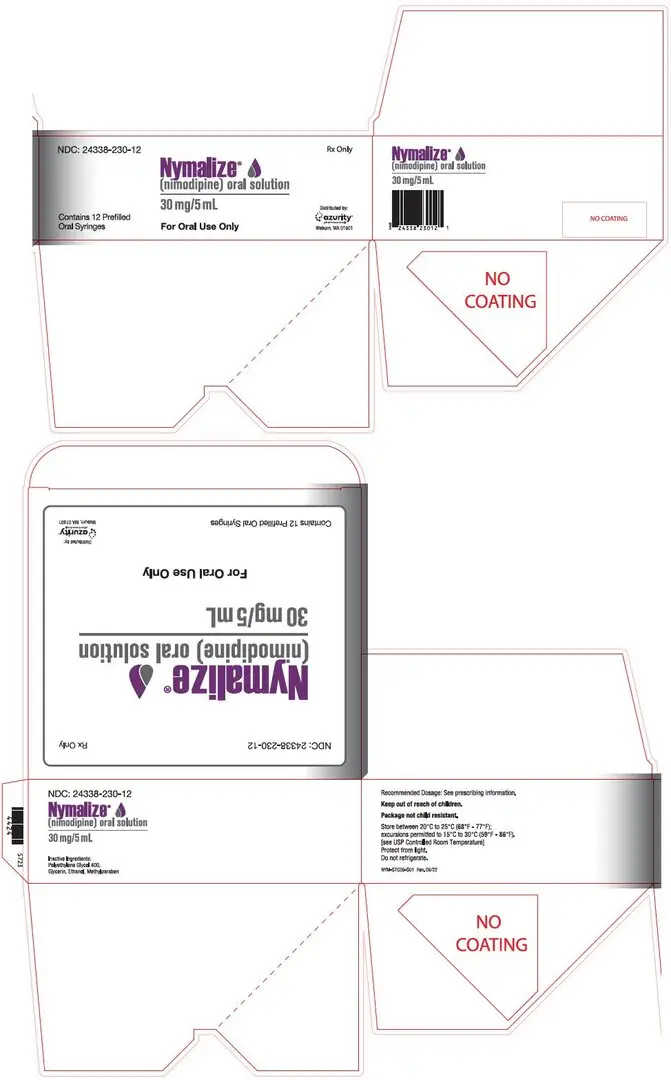
- 60 mg per 10 mL in unit-dose prefilled syringe (3)
- 30 mg per 5 mL in unit-dose prefilled syringe (3)
- 60 mg per 10 mL (6 mg/mL) in 8 oz bottle (3)
Contraindications
None (4)
Warnings and Precautions
- Hypotension: Monitor blood pressure. (5.1)
- Patients with Cirrhosis: Higher risk of adverse reactions. Monitor blood pressure and pulse. (5.2)
- CYP3A4 Strong Inhibitors: May significantly increase risk of hypotension. Concomitant use with NYMALIZE should generally be avoided. (5.3)
- CYP3A4 Strong Inducers: May significantly reduce efficacy of nimodipine. Concomitant use with NYMALIZE should generally be avoided. (5.4)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥1% and ≥1% placebo) were hypotension, headache, nausea, and bradycardia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Azurity Pharmaceuticals, Inc. at 1-800-461-7449 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Anti-Hypertensives: May increase risk of hypotension. Monitor blood pressure. (7.1)
- CYP3A4 Moderate and Weak Inhibitors: May increase risk of hypotension. Monitor blood pressure. Dose reduction of NYMALIZE may be needed. Avoid grapefruit juice. (7.2)
- CYP3A4 Moderate and Weak Inducers: May reduce efficacy of NYMALIZE. Dose increase may be needed. (7.3)
Use In Specific Populations
- Pregnancy: Based on animal data may cause fetal harm. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 5/2022
Full Prescribing Information
1. Indications and Usage for Nymalize
NYMALIZE is indicated for the improvement of neurological outcome by reducing the incidence and severity of ischemic deficits in adult patients with subarachnoid hemorrhage (SAH) from ruptured intracranial berry aneurysms regardless of their post-ictus neurological condition (i.e., Hunt and Hess Grades I-V).
2. Nymalize Dosage and Administration
2.1 Administration Instructions
Administer only enterally (e.g., oral, nasogastric tube, or gastric tube route). Do not administer intravenously or by other parenteral routes. For all routes of administration, begin NYMALIZE within 96 hours of the onset of SAH. Administer one hour before a meal or two hours after a meal for all routes of administration [see Clinical Pharmacology (12.3)].
2.2 Administration by Oral Route
The recommended oral dosage is 10 mL (60 mg) every 4 hours for 21 consecutive days.
2.3 Administration Via Nasogastric or Gastric Tube
Using the supplied prefilled oral syringe labeled "For Oral Use Only", administer 10 mL (60 mg) every 4 hours into a nasogastric or gastric tube for 21 consecutive days. For each dose, refill the syringe with 10 mL of 0.9% saline solution and then flush any remaining contents from nasogastric or gastric tube into the stomach.
3. Dosage Forms and Strengths
Oral Solution (6 mg per mL):
- 60 mg per 10 mL, pale yellow solution in unit-dose prefilled syringe
- 30 mg per 5 mL, pale yellow solution in unit-dose prefilled syringe
- 60 mg per 10 mL (6 mg/mL), pale yellow solution in 8 oz bottle
5. Warnings and Precautions
5.1 Hypotension
Blood pressure should be carefully monitored during treatment with NYMALIZE. In clinical studies of patients with subarachnoid hemorrhage, about 5% of nimodipine-treated patients compared to 1% of placebo-treated patients had hypotension and about 1% of nimodipine-treated patients left the study because of this [see Adverse Reactions (6)].
5.2 Possible Increased Risk of Adverse Reactions in Patients with Cirrhosis
Given that the plasma levels of nimodipine are increased in patients with cirrhosis, these patients are at higher risk of adverse reactions. Therefore, monitor blood pressure and pulse rate closely and administer a lower dosage [see Dosage and Administration (2.4), Clinical Pharmacology (12.3)].
5.3 Possible Increased Risk of Hypotension with Strong CYP3A4 Inhibitors
Concomitant use of strong inhibitors of CYP3A4, such as some macrolide antibiotics (e.g., clarithromycin, telithromycin), some HIV protease inhibitors (e.g., indinavir, nelfinavir, ritonavir, saquinavir), some HCV protease inhibitors (e.g., boceprevir, telaprevir), some azole antimycotics (e.g., ketoconazole, itraconazole, posaconazole, voriconazole), conivaptan, delavirdine, and nefazodone with nimodipine should generally be avoided because of a risk of significant hypotension [see Drug Interactions (7.2)].
5.4 Possible Reduced Efficacy with Strong CYP3A4 Inducers
Concomitant use of strong CYP3A4 inducers (e.g., carbamazepine, phenobarbital, phenytoin, rifampin, St. John's wort) and nimodipine should generally be avoided, as nimodipine plasma concentration and efficacy may be significantly reduced [see Drug Interactions (7.3)].
6. Adverse Reactions/Side Effects
The safety and efficacy of NYMALIZE (nimodipine oral solution) in the treatment of patients with SAH is based on adequate and well-controlled studies of nimodipine oral capsules in patients with SAH. NYMALIZE (nimodipine oral solution) has comparable bioavailability to nimodipine oral capsules.
The following clinically significant adverse reaction appears in other sections of the labeling:
- Hypotension [see Warnings and Precautions (5.1)].
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
In clinical trials of nimodipine oral capsules in patients with SAH, eleven percent (92 of 823) of nimodipine-treated patients reported adverse events compared to six percent (29 of 479) of placebo-treated patients. The most common adverse event was decreased blood pressure in 4.4% of nimodipine-treated patients. The events reported with a frequency greater than 1% are displayed in Table 1 by dose.
| Placebo (n=479) | Nimodipine dose every 4 hours | |||||
|---|---|---|---|---|---|---|
| 0.35 mg/kg (n=82) | 30 mg (n=71) | 60 mg (n=494) | 90 mg (n=172) | 120 mg (n=4) |
||
| Decreased Blood Pressure | 6 (1.2) | 1 (1.2) | 0 | 19 (3.8) | 14 (8.1) | 2 (50.0) |
| Edema | 3 (0.6) | 0 | 0 | 2 (0.4) | 2 (1.2) | 0 |
| Diarrhea | 3 (0.6) | 0 | 3 (4.2) | 0 | 3 (1.7) | 0 |
| Rash | 3 (0.6) | 2 (2.4) | 0 | 3 (0.6) | 2 (1.2) | 0 |
| Headache | 1 (0.2) | 0 | 1 (1.4) | 6 (1.2) | 0 | 0 |
| Gastrointestinal Symptoms | 0 | 2 (2.4) | 0 | 0 | 2 (1.2) | 0 |
| Nausea | 0 | 1 (1.2) | 1 (1.4) | 6 (1.2) | 1 (0.6) | 0 |
| Dyspnea | 0 | 1 (1.2) | 0 | 0 | 0 | 0 |
| EKG Abnormalities | 0 | 0 | 1 (1.4) | 0 | 1 (0.6) | 0 |
| Tachycardia | 0 | 0 | 1 (1.4) | 0 | 0 | 0 |
| Bradycardia | 0 | 0 | 0 | 5 (1.0) | 1 (0.6) | 0 |
| Muscle Pain/Cramp | 0 | 0 | 1 (1.4) | 1 (0.2) | 1 (0.6) | 0 |
| Acne | 0 | 0 | 1 (1.4) | 0 | 0 | 0 |
| Depression | 0 | 0 | 1 (1.4) | 0 | 0 | 0 |
SAH is frequently accompanied by alterations in consciousness that may lead to an under-reporting of adverse experiences. As a calcium channel blocker, nimodipine may have the potential to exacerbate heart failure in susceptible patients or to interfere with A-V conduction, but these events were not observed in SAH trials.
7. Drug Interactions
7.1 Blood Pressure Lowering Drugs
Nimodipine may increase the blood pressure lowering effect of concomitantly administered anti-hypertensives such as diuretics, beta-blockers, ACE inhibitors, angiotensin receptor blockers, other calcium channel blockers, α-adrenergic blockers, PDE5 inhibitors, and α-methyldopa. In Europe, nimodipine was observed to occasionally intensify the effect of antihypertensive drugs taken concomitantly by hypertensive patients; this phenomenon was not observed in North American clinical trials. Blood pressure should be carefully monitored, and dose adjustment of the blood pressure lowering drug(s) may be necessary.
7.2 CYP3A4 Inhibitors
Nimodipine plasma concentration can be significantly increased when concomitantly administered with strong CYP3A4 inhibitors. As a consequence, the blood pressure lowering effect may be increased. Therefore, the concomitant administration of NYMALIZE and strong CYP3A4 inhibitors should generally be avoided [see Warnings and Precautions (5.3)]. Strong CYP3A4 inhibitors include some members of the following classes:
- -
- macrolide antibiotics (e.g., clarithromycin, telithromycin),
- -
- HIV protease inhibitors (e.g., indinavir, nelfinavir, ritonavir, saquinavir),
- -
- HCV protease inhibitors (e.g., boceprevir, telaprevir),
- -
- azole antimycotics (e.g., ketoconazole, itraconazole, posaconazole, voriconazole),
- -
- conivaptan, delavirdine, nefazodone
Nimodipine plasma concentration can also be increased in the presence of moderate and weak inhibitors of CYP3A4. If nimodipine is concomitantly administered with these drugs, blood pressure should be monitored, and a reduction of the nimodipine dose may be necessary. Moderate and weak CYP3A4 inhibitors include alprozalam, ameprenavir, amiodarone, aprepitant, atazanavir, cimetidine, cyclosporine, diltiazem, erythromycin, fluconazole, fluoxetine, isoniazid, oral contraceptives, quinuprestin/dalfopristin, valproic acid, and verapamil.
A study in eight healthy volunteers has shown a 50% increase in mean peak nimodipine plasma concentrations and a 90% increase in mean area under the curve, after a one-week course of cimetidine at 1,000 mg/day and nimodipine at 90 mg/day. This effect may be mediated by the known inhibition of hepatic cytochrome P-450 (CYP) by cimetidine, which could decrease first-pass metabolism of nimodipine.
Grapefruit juice inhibits CYP3A4. Ingestion of grapefruit/grapefruit juice is not recommended while taking nimodipine.
7.3 CYP3A4 Inducers
Nimodipine plasma concentration and efficacy may be significantly reduced when concomitantly administered with strong CYP3A4 inducers. Therefore, concomitant use of NYMALIZE with strong CYP3A4 inducers (e.g., carbamazepine, phenobarbital, phenytoin, rifampin, St. John's wort) should generally be avoided [see Warnings and Precautions (5.4)].
Moderate and weak inducers of CYP3A4 may also reduce the efficacy of nimodipine. Patients on these should be closely monitored for lack of effectiveness, and a nimodipine dosage increase may be required. Moderate and weak CYP3A4 inducers include, for example: amprenavir, aprepitant, armodafinil, bosentan, efavirnenz, etravirine, Echinacea, modafinil, nafcillin, pioglitazone, prednisone, rufinamide, and vemurafenib.
8. Use In Specific Populations
8.5 Geriatric Use
Clinical studies of nimodipine did not include sufficient numbers of subjects aged 65 and over to determine whether they had a different clinical response than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dosing in elderly patients should be cautious, reflecting the greater frequency of decreased hepatic, renal or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
10. Overdosage
There have been no reports of overdosage from the oral administration of nimodipine. Symptoms of overdosage would be expected to be related to cardiovascular effects such as excessive peripheral vasodilation with marked systemic hypotension. Clinically significant hypotension due to nimodipine overdosage may require active cardiovascular support with pressor agents and specific treatments for calcium channel blocker overdose. Since nimodipine is highly protein-bound, dialysis is not likely to be of benefit.
11. Nymalize Description
NYMALIZE contains nimodipine, a dihydropyridine calcium channel blocker. Nimodipine is isopropyl 2-methoxyethyl 1,4-dihydro-2,6-dimethyl-4-(m-nitrophenyl)-3,5-pyridinedicarboxylate. It has a molecular weight of 418.5 and a molecular formula of C21H26N2O7. The structural formula is:
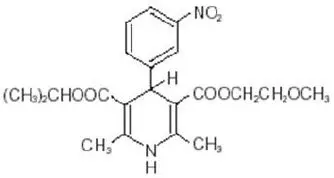
Nimodipine is a yellow crystalline substance, practically insoluble in water.
NYMALIZE Oral Solution contains 60 mg of nimodipine per 10 mL. In addition, the oral solution contains the following inactive ingredients: ethanol, glycerin, methylparaben, polyethylene glycol 400.
12. Nymalize - Clinical Pharmacology
12.1 Mechanism of Action
Nimodipine is a dihydropyridine calcium channel blocker. The contractile processes of smooth muscle cells are dependent upon calcium ions, which enter these cells during depolarization as slow ionic transmembrane currents. Nimodipine inhibits calcium ion transfer into these cells and thus inhibits contractions of vascular smooth muscle. In animal experiments, nimodipine had a greater effect on cerebral arteries than on arteries elsewhere in the body perhaps because it is highly lipophilic, allowing it to cross the blood-brain barrier; concentrations of nimodipine as high as 12.5 ng/mL have been detected in the cerebrospinal fluid of nimodipine-treated SAH patients.
The precise mechanism of action of nimodipine in reducing the incidence and severity of ischemic deficits in adult patients with SAH from ruptured intracranial berry aneurysms is unknown. Although the clinical studies demonstrate a favorable effect of nimodipine on the severity of neurological deficits caused by cerebral vasospasm following SAH, there is no arteriographic evidence that nimodipine either prevents or relieves the spasm of these arteries. However, whether or not the arteriographic methodology utilized was adequate to detect a clinically meaningful effect, if any, on vasospasm is unknown.
12.3 Pharmacokinetics
After a single 60 mg oral dose of NYMALIZE, mean (CV%) Cmax was 69.9 ng/mL (36.1%), AUCinf was 151 h∙ng/mL (36.0%) and within subject variability (CV%) was 21.7% and 12.4%, respectively. There were no signs of accumulation when nimodipine was given three times a day for seven days.
14. Clinical Studies
The safety and efficacy of NYMALIZE (nimodipine oral solution) in the treatment of patients with SAH is based on adequate and well-controlled studies of nimodipine oral capsules in patients with SAH. NYMALIZE (nimodipine oral solution) has comparable bioavailability to nimodipine oral capsules.
Nimodipine has been shown in 4 randomized, double-blind, placebo-controlled trials to reduce the severity of neurological deficits resulting from vasospasm in patients who have had a recent SAH (Studies 1, 2, 3, and 4).
The trials used doses ranging from 20-30 mg to 90 mg every 4 hours, with drug given for 21 days in 3 studies, and for at least 18 days in the other. Three of the four trials followed patients for 3-6 months. Three of the trials studied relatively well patients, with all or most patients in Hunt and Hess Grades I - III (essentially free of focal deficits after the initial bleed). Study 4 studied much sicker patients with Hunt and Hess Grades III - V. Studies 1 and 2 were similar in design, with relatively unimpaired SAH patients randomized to nimodipine or placebo. In each, a judgment was made as to whether any late-developing deficit was due to spasm or other causes, and the deficits were graded. Both studies showed significantly fewer severe deficits due to spasm in the nimodipine group; Study 2 showed fewer spasm-related deficits of all severities. No effect was seen on deficits not related to spasm. See Table 2.
| Study | Grade* | Treatment | Patients | ||
|---|---|---|---|---|---|
| Number Analyzed | Number of Patients with Any Deficit Due to Spasm | Numbers with Severe Deficit | |||
|
|||||
| Study 1 | I-III | Nimodipine 20-30 mg every 4 hours | 56 | 13 | 1 |
| Placebo | 60 | 16 | 8† | ||
| Study 2 | I-III | Nimodipine 60 mg every 4 hours | 31 | 4 | 2 |
| Placebo | 39 | 11 | 10† | ||
Study 3 was a 554-patient trial that included SAH patients with all grades of severity (89% were in Hunt and Hess Grades I-III). In Study 3, patients were treated with placebo or 60 mg of nimodipine every 4 hours. Outcomes were not defined as spasm related or not but there was a significant reduction in the overall rate of brain infarction and severely disabling neurological outcome at 3 months (Table 3):
| Nimodipine | Placebo | |
|---|---|---|
|
||
| Total patients | 278 | 276 |
| Good recovery | 199* | 169 |
| Moderate disability | 24 | 16 |
| Severe disability | 12† | 31 |
| Death | 43‡ | 60 |
Study 4 enrolled much sicker patients, (Hunt and Hess Grades III-V), who had a high rate of death and disability, and used a dose of 90 mg every 4 hours, but was otherwise similar to Study 1 and Study 2. Analysis of delayed ischemic deficits, many of which result from spasm, showed a significant reduction in spasm-related deficits. Among analyzed patients (72 nimodipine, 82 placebo), there were the following outcomes (Table 4).
| Delayed Ischemic Deficits (DID) | Permanent Deficits | |||
|---|---|---|---|---|
| Nimodipine 90 mg every 4 hours | Placebo | Nimodipine 90 mg every 4 hours | Placebo | |
| n (%) | n (%) | n (%) | n (%) | |
|
||||
| DID Spasm Alone | 8 (11)* | 25 (31) | 5 (7)* | 22 (27) |
| DID Spasm Contributing | 18 (25) | 21 (26) | 16 (22) | 17 (21) |
| DID Without Spasm | 7 (10) | 8 (10) | 6 (8) | 7 (9) |
| No DID | 39 (54) | 28 (34) | 45 (63) | 36 (44) |
When data were combined for Study 3 and Study 4, the treatment difference on success rate (i.e., good recovery) on the Glasgow Outcome Scale was 25.3% (nimodipine) versus 10.9% (placebo) for Hunt and Hess Grades IV or V. Table 5 demonstrates that nimodipine tends to improve good recovery of SAH patients with poor neurological status post-ictus, while decreasing the numbers with severe disability and vegetative survival.
| Glasgow Outcome* | Nimodipine (n=87) | Placebo (n=101) |
|---|---|---|
|
||
| Good Recovery | 22 (25.3%) | 11 (10.9%) |
| Moderate Disability | 8 (9.2%) | 12 (11.9%) |
| Severe Disability | 6 (6.9%) | 15 (14.9%) |
| Vegetative Survival | 4 (4.6%) | 9 (8.9%) |
| Death | 47 (54.0%) | 54 (53.5%) |
A dose-ranging study comparing 30 mg, 60 mg, and 90 mg doses found a generally low rate of spasm-related neurological deficits but no dose response relationship.
16. How is Nymalize supplied
NYMALIZE (nimodipine) Oral Solution 6 mg/mL is a pale yellow solution and is supplied as follows:
- NDC 24338-260-08: 8 oz. bottle (237 mL) 60 mg/10 mL (6 mg/mL)
- NDC 24338-260-12: Carton containing 12 individually wrapped 10 mL packages.
Each package contains one 60 mg/10 mL unit-dose prefilled oral syringe with a purple plunger (NDC 24338-260-10). - NDC 24338-230-12: Carton containing 12 individually wrapped 5 mL packages.
Each package contains one 30 mg/5 mL unit-dose prefilled oral syringe with a white plunger (NDC 24338-230-05).
17. Patient Counseling Information
Inform patients that the most frequent adverse reaction associated with nimodipine is decreased blood pressure [see Warnings and Precautions (5.1)]. Inform them that use of NYMALIZE with anti-hypertensives can cause increased drop in blood pressure [see Drug Interactions (7.1)].
Patients should be aware that ingestion of grapefruit or grapefruit juice should be avoided when taking NYMALIZE due to its ability to increase nimodipine plasma concentrations and potential to increase the risk of hypotension [see Drug Interactions (7.2)].
Advise patients to notify their healthcare provider if they become pregnant during treatment or plan to become pregnant during therapy [see Use in Specific Populations (8.1)].
Advise female patients to notify their physicians if they intend to breastfeed or are breastfeeding an infant [see Use in Specific Populations (8.2)].
| NYMALIZE
nimodipine solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| NYMALIZE
nimodipine solution |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Azurity Pharmaceuticals, Inc. (117505635) |