Drug Detail:Olopatadine (monograph) (Pataday)
Drug Class:
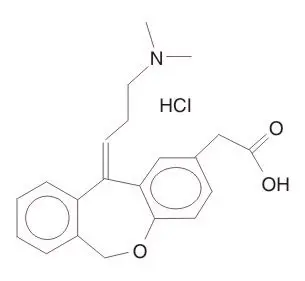
Olopatadine Ophthalmic Solution Description
Chemical Name: 11-[(Z)-3-(Dimethylamino)propylidene]-6-11-dihydrodibenz[b,e] oxepin-2-acetic acid hydrochloride
Olopatadine Ophthalmic Solution - Clinical Pharmacology
Results from an environmental study demonstrated that Olopatadine hydrochloride ophthalmic solution was effective in the treatment of the signs and symptoms of allergic conjunctivitis when dosed twice daily for up to 6 weeks. Results from conjunctival antigen challenge studies demonstrated that Olopatadine hydrochloride ophthalmic solution, when subjects were challenged with antigen both initially and up to 8 hours after dosing, was significantly more effective than its vehicle in preventing ocular itching associated with allergic conjunctivitis.
Precautions
Information for Patients :
Patients should be advised not to wear a contact lens if their eye is red. Olopatadine hydrochloride ophthalmic solution, 0.1% should not be used to treat contact lens related irritation. The preservative in Olopatadine hydrochloride ophthalmic solution, 0.1% , benzalkonium chloride, may be absorbed by soft contact lenses. Patients who wear soft contact lenses and whose eyes are not red should be instructed to wait at least ten minutes after instilling Olopatadine hydrochloride ophthalmic solution, 0.1% before they insert their contact lenses.
Pregnancy:
Nursing Mothers :
Olopatadine has been identified in the milk of nursing rats following oral administration. It is not known whether topical ocular administration could result in sufficient systemic absorption to produce detectable quantities in the human breast milk. Nevertheless, caution should be exercised when Olopatadine hydrochloride ophthalmic solution, 0.1% is administered to a nursing mother.
Pediatric Use:
Safety and effectiveness in pediatric patients below the age of 3 years have not been established.
Geriatric Use:
No overall differences in safety or effectiveness have been observed between elderly and younger patients.
Adverse Reactions/Side Effects
To report SUSPECTED ADVERSE REACTIONS, contact FDA at 1-800-FDA-1088 or www.fda.gov/medwatch
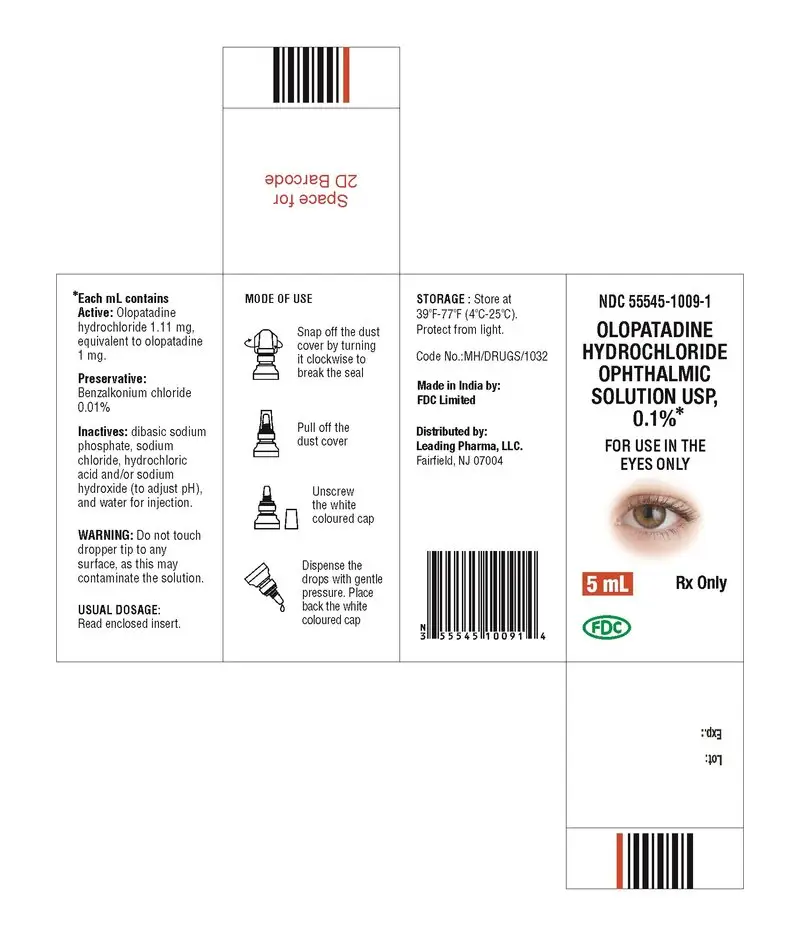
How is Olopatadine Ophthalmic Solution supplied
5 mL: NDC 55545-1009-1
Storage: Store at 39°F-77°F (4°C-25°C). Protect from light.
Rx Only
Made in India by:
FDC Limited
B-8, MIDC Industrial Area,
Waluj, Aurangabad 431 136, Maharashtra
India.
Distributed by:
Leading Pharma LLC
Fairfield, NJ07004
Revised: October 2019
| OLOPATADINE
olopatadine solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - FDC Limited (650441301) |
| Registrant - FDC Limited (650078413) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| FDC Limited | 862267994 | manufacture(55545-1009) , analysis(55545-1009) | |