Drug Detail:Olysio (Simeprevir [ sim-e-pre-vir ])
Drug Class: Protease inhibitors
Highlights of Prescribing Information
OLYSIO (simeprevir) capsules, for oral use
Initial U.S. Approval: 2013
WARNING: RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH HCV AND HBV
See full prescribing information for complete boxed warning.
Hepatitis B virus (HBV) reactivation has been reported, in some cases resulting in fulminant hepatitis, hepatic failure, and death. (5.1)
Recent Major Changes
| Boxed warning | 02/2017 |
| Dosage and Administration (2.1) | 02/2017 |
| Warnings and Precautions (5.1) | 02/2017 |
Indications and Usage for Olysio
OLYSIO is a hepatitis C virus (HCV) NS3/4A protease inhibitor indicated for the treatment of adults with chronic hepatitis C virus (HCV) infection:
- in combination with sofosbuvir in patients with HCV genotype 1 without cirrhosis or with compensated cirrhosis
- in combination with peginterferon alfa (Peg-IFN-alfa) and ribavirin (RBV) in patients with HCV genotype 1 or 4 without cirrhosis or with compensated cirrhosis. (1)
Limitations of Use:
- Efficacy of OLYSIO in combination with Peg-IFN-alfa and RBV is substantially reduced in patients infected with HCV genotype 1a with an NS3 Q80K polymorphism. (2.1, 12.4)
- OLYSIO is not recommended in patients who have previously failed therapy with a treatment regimen that included OLYSIO or other HCV protease inhibitors. (1, 12.4)
Olysio Dosage and Administration
- Testing Prior to the Initiation of Therapy:
- Test all patients for HBV infection by measuring HBsAg and anti-HBc. (2.1)
- Prior to initiation of treatment with OLYSIO in combination with Peg-IFN-alfa and RBV in patients infected with HCV genotype 1a, screening for the presence of virus with the NS3 Q80K polymorphism is strongly recommended and alternative therapy should be considered if Q80K is detected. (2.1, 12.4)
- Monitor liver chemistry tests before and during OLYSIO combination therapy. (2.1, 5.3)
- Recommended dosage: One 150 mg capsule taken once daily with food. (2.2)
Treatment Regimens and Duration by Patient Population Patient population Treatment regimen Duration - *
- followed by 12 or 36 additional weeks of Peg-IFN-alfa + RBV depending on prior response status and presence of HIV-1 co-infection. (2.2)
Genotype 1
without cirrhosisOLYSIO + sofosbuvir 12 weeks Genotype 1
with compensated cirrhosis (Child-Pugh A)OLYSIO + sofosbuvir 24 weeks Genotype 1 or 4
without cirrhosis or with compensated cirrhosis (Child-Pugh A), with or without HIV-1 co-infectionOLYSIO + Peg-IFN-alfa + RBV 12 weeks*
- Refer to the Full Prescribing Information for details on stopping rules when discontinuing OLYSIO in combination with Peg-IFN-alfa + RBV and for information on dosing adjustment and interruption. (2.3, 2.4)
- OLYSIO is not recommended in patients with moderate or severe hepatic impairment (Child–Pugh B or C). (2.5)
Dosage Forms and Strengths
Capsules: 150 mg (3)
Contraindications
Because OLYSIO is used only in combination with other antiviral drugs (including Peg-IFN-alfa and RBV) for the treatment of chronic HCV infection, the contraindications to other drugs also apply to the combination regimen. (4)
Warnings and Precautions
- Risk of Hepatitis B Virus Reactivation: Test all patients for evidence of current or prior HBV infection before initiation of HCV treatment. Monitor HCV/HBV coinfected patients for HBV reactivation and hepatitis flare during HCV treatment and post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated. (5.1)
- Serious Symptomatic Bradycardia with Sofosbuvir and Amiodarone coadministration: Serious symptomatic bradycardia may occur in patients taking amiodarone with sofosbuvir in combination with OLYSIO, particularly in patients also receiving beta blockers, or those with underlying cardiac comorbidities and/or advanced liver disease. Coadministration of amiodarone with OLYSIO in combination with sofosbuvir is not recommended. In patients without alternative treatment options, cardiac monitoring is recommended. (5.2, 6.2, 7.3)
- Hepatic Decompensation and Hepatic Failure: Hepatic decompensation and hepatic failure, including fatal cases have been reported in patients with advanced and/or decompensated cirrhosis. Monitor liver chemistry tests before and during OLYSIO combination therapy. (5.3)
- Photosensitivity: Serious photosensitivity reactions have been observed during OLYSIO combination therapy. Use sun protection measures and limit sun exposure during OLYSIO combination therapy. Consider discontinuation if a photosensitivity reaction occurs. (5.5)
- Rash: Rash has been observed during OLYSIO combination therapy. Discontinue OLYSIO if severe rash occurs. (5.6)
Adverse Reactions/Side Effects
- Most common adverse events reported with OLYSIO with sofosbuvir during 12 or 24 weeks of treatment: fatigue, headache and nausea. (6.1)
- Most common adverse reactions reported with OLYSIO with Peg-IFN-alfa and RBV during first 12 weeks of treatment: rash (including photosensitivity), pruritus and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Products, LP at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Coadministration of amiodarone with sofosbuvir in combination with OLYSIO may result in serious symptomatic bradycardia. (5.2, 7.3)
- Coadministration of OLYSIO with drugs that are moderate or strong inducers or inhibitors of CYP3A may significantly affect the plasma concentrations of simeprevir. The potential for drug-drug interactions must be considered prior to and during treatment. (5.8, 7, 12.3)
- Close monitoring of international normalized ratio (INR) values is recommended in patients receiving warfarin. (7.1)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2017
Related/similar drugs
Epclusa, Mavyret, Harvoni, sofosbuvir / velpatasvir, Sovaldi, VoseviFull Prescribing Information
WARNING: RISK OF HEPATITIS B VIRUS REACTIVATION IN PATIENTS COINFECTED WITH HCV AND HBV
Test all patients for evidence of current or prior hepatitis B virus (HBV) infection before initiating treatment with OLYSIO. HBV reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct-acting antivirals and were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Monitor HCV/HBV coinfected patients for hepatitis flare or HBV reactivation during HCV treatment and post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated [see Warnings and Precautions (5.1)].
1. Indications and Usage for Olysio
OLYSIO® is indicated for the treatment of adults with chronic hepatitis C virus (HCV) infection [see Dosage and Administration (2.2) and Clinical Studies (14)]:
- in combination with sofosbuvir in patients with HCV genotype 1 without cirrhosis or with compensated cirrhosis
- in combination with peginterferon alfa (Peg-IFN-alfa) and ribavirin (RBV) in patients with HCV genotype 1 or 4 without cirrhosis or with compensated cirrhosis.
2. Olysio Dosage and Administration
2.2 OLYSIO Combination Treatment
Administer OLYSIO in combination with other antiviral drugs for the treatment of chronic HCV infection. OLYSIO monotherapy is not recommended. The recommended dosage of OLYSIO is one 150 mg capsule taken orally once daily with food [see Clinical Pharmacology (12.3)]. The capsule should be swallowed as a whole. For specific dosing recommendations for the antiviral drugs used in combination with OLYSIO, refer to their respective prescribing information.
OLYSIO can be taken in combination with sofosbuvir or in combination with Peg-IFN-alfa and RBV.
OLYSIO in Combination with Sofosbuvir
Table 1 displays the recommended treatment regimen and duration of OLYSIO in combination with sofosbuvir in patients with chronic HCV genotype 1 infection.
| Patient Population (HCV Genotype 1) | Treatment Regimen and Duration |
|---|---|
|
|
| Treatment-naïve and treatment-experienced* patients: | |
| without cirrhosis | 12 weeks of OLYSIO + sofosbuvir |
| with compensated cirrhosis (Child-Pugh A) | 24 weeks of OLYSIO + sofosbuvir |
OLYSIO in Combination with Peg-IFN-alfa and RBV
Table 2 displays the recommended treatment regimen and duration of OLYSIO in combination with Peg-IFN-alfa and RBV in mono-infected and HCV/HIV-1 co-infected patients with HCV genotype 1 or 4 infection. Refer to Table 3 for treatment stopping rules for OLYSIO combination therapy with Peg-IFN-alfa and RBV.
| Patient Population (HCV Genotype 1 or 4) | Treatment Regimen and Duration |
|---|---|
| HIV = human immunodeficiency virus. | |
|
|
| Treatment-naïve patients and prior relapsers*: | |
| HCV mono-infected patients without cirrhosis or with compensated cirrhosis (Child-Pugh A) | 12 weeks of OLYSIO + Peg-IFN-alfa + RBV followed by additional 12 weeks of Peg-IFN-alfa + RBV (total treatment duration of 24 weeks)† |
| HCV/HIV-1 co-infected patients without cirrhosis | |
| HCV/HIV-1 co-infected patients with compensated cirrhosis (Child-Pugh A) | 12 weeks of OLYSIO + Peg-IFN-alfa + RBV followed by additional 36 weeks of Peg-IFN-alfa + RBV (total treatment duration of 48 weeks)† |
| Prior non-responders (including partial‡ and null responders§): | |
| HCV/HIV-1 co-infected or HCV mono-infected patients without cirrhosis or with compensated cirrhosis (Child-Pugh A) | 12 weeks of OLYSIO + Peg-IFN-alfa + RBV followed by additional 36 weeks of Peg-IFN-alfa + RBV (total treatment duration of 48 weeks)† |
2.3 Discontinuation of Dosing
OLYSIO in Combination with Peg-IFN-alfa and RBV
During treatment, HCV RNA levels should be monitored as clinically indicated using a sensitive assay with a lower limit of quantification of at least 25 IU/mL. Because patients with an inadequate on-treatment virologic response (i.e., HCV RNA greater or equal to 25 IU/mL) are not likely to achieve a sustained virologic response (SVR), discontinuation of treatment is recommended in these patients. Table 3 presents treatment stopping rules for patients who experience an inadequate on-treatment virologic response at Weeks 4, 12, and 24.
| Treatment Week | HCV RNA | Action |
|---|---|---|
| Week 4 | ≥ 25 IU/mL | Discontinue OLYSIO, Peg-IFN-alfa, and RBV |
| Week 12 | Discontinue Peg-IFN-alfa, and RBV (treatment with OLYSIO is complete at Week 12) | |
| Week 24 | Discontinue Peg-IFN-alfa, and RBV (treatment with OLYSIO is complete at Week 12) |
2.4 Dosage Adjustment or Interruption
To prevent treatment failure, avoid reducing the dosage of OLYSIO or interrupting treatment. If treatment with OLYSIO is discontinued because of adverse reactions or inadequate on-treatment virologic response, OLYSIO treatment must not be reinitiated [see Warnings and Precautions (5.3)].
If adverse reactions potentially related to the antiviral drug(s) used in combination with OLYSIO occur, refer to the instructions outlined in their respective prescribing information for recommendations on dosage adjustment or interruption.
If any of the other antiviral drug(s) used in combination with OLYSIO for the treatment of chronic HCV infection are permanently discontinued for any reason, OLYSIO should also be discontinued.
2.5 Not Recommended in Patients with Moderate or Severe Hepatic Impairment
OLYSIO is not recommended for patients with moderate or severe hepatic impairment (Child-Pugh B or C) [see Warnings and Precautions (5.3), Adverse Reactions (6.1), Use in Specific Populations (8.8), and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
OLYSIO is available as a white gelatin capsule marked with "TMC435 150" in black ink. Each capsule contains 150 mg simeprevir.
4. Contraindications
Because OLYSIO is used only in combination with other antiviral drugs (including Peg-IFN-alfa and RBV) for the treatment of chronic HCV infection, the contraindications to other drugs also apply to the combination regimen. Refer to the respective prescribing information for a list of contraindications.
5. Warnings and Precautions
5.1 Risk of Hepatitis B Virus Reactivation in Patients Coinfected with HCV and HBV
Hepatitis B virus (HBV) reactivation has been reported in HCV/HBV coinfected patients who were undergoing or had completed treatment with HCV direct-acting antivirals, and who were not receiving HBV antiviral therapy. Some cases have resulted in fulminant hepatitis, hepatic failure, and death. Cases have been reported in patients who are HBsAg positive and also in patients with serologic evidence of resolved HBV infection (i.e., HBsAg negative and anti-HBc positive). HBV reactivation has also been reported in patients receiving certain immunosuppressant or chemotherapeutic agents; the risk of HBV reactivation associated with treatment with HCV direct-acting antivirals may be increased in these patients.
HBV reactivation is characterized as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA level. In patients with resolved HBV infection, reappearance of HBsAg can occur. Reactivation of HBV replication may be accompanied by hepatitis, i.e., increases in aminotransferase levels and, in severe cases, increases in bilirubin levels, liver failure, and death can occur.
Test all patients for evidence of current or prior HBV infection by measuring HBsAg and anti-HBc before initiating HCV treatment with OLYSIO. In patients with serologic evidence of HBV infection, monitor for clinical and laboratory signs of hepatitis flare or HBV reactivation during HCV treatment with OLYSIO and during post-treatment follow-up. Initiate appropriate patient management for HBV infection as clinically indicated.
5.2 Serious Symptomatic Bradycardia When Coadministered with Sofosbuvir and Amiodarone
Postmarketing cases of symptomatic bradycardia and cases requiring pacemaker intervention have been reported when amiodarone was coadministered with a sofosbuvir-containing regimen. A fatal cardiac arrest was reported in a patient taking amiodarone who was coadministered a sofosbuvir-containing regimen (ledipasvir/sofosbuvir). Bradycardia has generally occurred within hours to days, but cases have been observed up to 2 weeks after initiating HCV treatment. Patients also taking beta blockers, or those with underlying cardiac comorbidities and/or advanced liver disease may be at increased risk for symptomatic bradycardia with coadministration of amiodarone. Bradycardia generally resolved after discontinuation of HCV treatment. The mechanism for this effect is unknown.
Coadministration of amiodarone with OLYSIO in combination with sofosbuvir is not recommended. For patients taking amiodarone who have no other alternative treatment options, and who will be coadministered OLYSIO and sofosbuvir:
- Counsel patients about the risk of serious symptomatic bradycardia.
- Cardiac monitoring in an in-patient setting for the first 48 hours of coadministration is recommended, after which outpatient or self-monitoring of the heart rate should occur on a daily basis through at least the first 2 weeks of treatment.
Patients who are taking sofosbuvir in combination with OLYSIO who need to start amiodarone therapy due to no other alternative treatment options should undergo similar cardiac monitoring as outlined above.
Due to amiodarone's long elimination half-life, patients discontinuing amiodarone just prior to starting sofosbuvir in combination with OLYSIO should also undergo similar cardiac monitoring as outlined above.
Patients who develop signs or symptoms of bradycardia should seek medical evaluation immediately. Symptoms may include near-fainting or fainting, dizziness or lightheadedness, malaise, weakness, excessive tiredness, shortness of breath, chest pain, confusion or memory problems [see Adverse Reactions (6.2) and Drug Interactions (7.3)].
5.3 Hepatic Decompensation and Hepatic Failure
Hepatic decompensation and hepatic failure, including fatal cases, have been reported postmarketing in patients treated with OLYSIO in combination with Peg-IFN-alfa and RBV or in combination with sofosbuvir. Most cases were reported in patients with advanced and/or decompensated cirrhosis who are at increased risk for hepatic decompensation or hepatic failure. Because these events have been reported voluntarily during clinical practice, estimates of frequency cannot be made; and a causal relationship between treatment with OLYSIO and these events has not been established [see Adverse Reactions (6.2)].
OLYSIO is not recommended for patients with moderate or severe hepatic impairment (Child-Pugh B or C) [see Dosage and Administration (2.5) and Use in Specific Populations (8.8)].
In clinical trials of OLYSIO, modest increases in bilirubin levels were observed without impacting hepatic function [see Adverse Reactions (6.1)]. Postmarketing cases of hepatic decompensation with markedly elevated bilirubin levels have been reported. Monitor liver chemistry tests before and as clinically indicated during OLYSIO combination therapy. Patients who experience an increase in total bilirubin to greater than 2.5 times the upper limit of normal should be closely monitored:
- Patients should be instructed to contact their healthcare provider if they have onset of fatigue, weakness, lack of appetite, nausea and vomiting, jaundice or discolored feces.
- Discontinue OLYSIO if elevation in bilirubin is accompanied by liver transaminase increases or clinical signs and symptoms of hepatic decompensation.
5.4 Risk of Serious Adverse Reactions Associated with Combination Treatment
Because OLYSIO is used in combination with other antiviral drugs for the treatment of chronic HCV infection, consult the prescribing information for these drugs before starting therapy with OLYSIO. Warnings and Precautions related to these drugs also apply to their use in OLYSIO combination treatment.
5.5 Photosensitivity
Photosensitivity reactions have been observed with OLYSIO combination therapy. Serious photosensitivity reactions resulting in hospitalization have been observed with OLYSIO in combination with Peg-IFN-alfa and RBV [see Adverse Reactions (6.1)]. Photosensitivity reactions occurred most frequently in the first 4 weeks of treatment, but can occur at any time during treatment. Photosensitivity may present as an exaggerated sunburn reaction, usually affecting areas exposed to light (typically the face, "V" area of the neck, extensor surfaces of the forearms, and dorsa of the hands). Manifestations may include burning, erythema, exudation, blistering, and edema.
Use sun protective measures and limit sun exposure during treatment with OLYSIO. Avoid use of tanning devices during treatment with OLYSIO. Discontinuation of OLYSIO should be considered if a photosensitivity reaction occurs and patients should be monitored until the reaction has resolved. If a decision is made to continue OLYSIO in the setting of a photosensitivity reaction, expert consultation is advised.
5.6 Rash
Rash has been observed with OLYSIO combination therapy [see Adverse Reactions (6.1)]. Rash occurred most frequently in the first 4 weeks of treatment, but can occur at any time during treatment. Severe rash and rash requiring discontinuation of OLYSIO have been reported in subjects receiving OLYSIO in combination with Peg-IFN-alfa and RBV. Most of the rash events in OLYSIO-treated patients were of mild or moderate severity [see Adverse Reactions (6.1)]. Patients with mild to moderate rashes should be followed for possible progression of rash, including the development of mucosal signs (e.g., oral lesions, conjunctivitis) or systemic symptoms. If the rash becomes severe, OLYSIO should be discontinued. Patients should be monitored until the rash has resolved.
5.7 Sulfa Allergy
OLYSIO contains a sulfonamide moiety. In subjects with a history of sulfa allergy (n=16), no increased incidence of rash or photosensitivity reactions has been observed. However, there are insufficient data to exclude an association between sulfa allergy and the frequency or severity of adverse reactions observed with the use of OLYSIO.
5.8 Risk of Adverse Reactions or Reduced Therapeutic Effect Due to Drug Interactions
Coadministration of OLYSIO with substances that are moderate or strong inducers or inhibitors of cytochrome P450 3A (CYP3A) is not recommended as this may lead to significantly lower or higher exposure of simeprevir, respectively, which may result in reduced therapeutic effect or adverse reactions [see Drug Interactions (7) and Clinical Pharmacology (12.3)].
6. Adverse Reactions/Side Effects
Because OLYSIO is administered in combination with other antiviral drugs, refer to the prescribing information of the antiviral drugs used in combination with OLYSIO for a description of adverse reactions associated with their use.
The following serious and otherwise important adverse reactions are described below and in other sections of the labeling:
- Serious Symptomatic Bradycardia When Coadministered with Sofosbuvir and Amiodarone [see Warnings and Precautions (5.2) and Drug Interactions (7.3)]
- Hepatic Decompensation and Hepatic Failure [see Warnings and Precautions (5.3)]
- Photosensitivity [see Warnings and Precautions (5.5)]
- Rash [see Warnings and Precautions (5.6)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
OLYSIO in Combination with Peg-IFN-alfa and RBV
The safety profile of OLYSIO in combination with Peg-IFN-alfa and RBV in patients with HCV genotype 1 infection is based on pooled data from three Phase 3 trials (QUEST-1, QUEST-2 and PROMISE) [see Clinical Studies (14.3)]. These trials included a total of 1178 subjects who received OLYSIO or placebo in combination with 24 or 48 weeks of Peg-IFN-alfa and RBV. Of the 1178 subjects, 781 subjects were randomized to receive OLYSIO 150 mg once daily for 12 weeks and 397 subjects were randomized to receive placebo once daily for 12 weeks.
In the pooled Phase 3 safety data, the majority of the adverse reactions reported during 12 weeks treatment with OLYSIO in combination with Peg-IFN-alfa and RBV were Grade 1 to 2 in severity. Grade 3 or 4 adverse reactions were reported in 23% of subjects receiving OLYSIO in combination with Peg-IFN-alfa and RBV versus 25% of subjects receiving placebo in combination with Peg-IFN-alfa and RBV. Serious adverse reactions were reported in 2% of subjects receiving OLYSIO in combination with Peg-IFN-alfa and RBV and in 3% of subjects receiving placebo in combination with Peg-IFN-alfa and RBV. Discontinuation of OLYSIO or placebo due to adverse reactions occurred in 2% and 1% of subjects receiving OLYSIO with Peg-IFN-alfa and RBV and subjects receiving placebo with Peg-IFN-alfa and RBV, respectively.
Table 6 lists adverse reactions (all Grades) that occurred with at least 3% higher frequency among subjects with HCV genotype 1 infection receiving OLYSIO 150 mg once daily in combination with Peg-IFN-alfa and RBV, compared to subjects receiving placebo in combination with Peg-IFN-alfa and RBV, during the first 12 weeks of treatment in the pooled Phase 3 trials in subjects who were treatment-naïve or who had previously relapsed after Peg-IFN-alfa and RBV therapy.
| Adverse Reaction‡ | OLYSIO 150 mg + Peg-IFN-alfa+ RBV First 12 Weeks N=781 % (n) | Placebo + Peg-IFN-alfa+ RBV First 12 Weeks N=397 % (n) |
|---|---|---|
|
||
| Rash (including photosensitivity) | 28 (218) | 20 (79) |
| Pruritus | 22 (168) | 15 (58) |
| Nausea | 22 (173) | 18 (70) |
| Myalgia | 16 (126) | 13 (53) |
| Dyspnea | 12 (92) | 8 (30) |
Laboratory Abnormalities
Among subjects who received OLYSIO or placebo plus Peg-IFN-alfa and RBV, there were no differences between treatment groups for the following laboratory parameters: hemoglobin, neutrophils, platelets, aspartate aminotransferase, alanine aminotransferase, amylase, or serum creatinine. Laboratory abnormalities that were observed at a higher incidence in OLYSIO-treated subjects than in placebo-treated subjects are listed in Table 7.
| Laboratory Parameter | WHO Toxicity Range | OLYSIO 150 mg + Peg-IFN-alfa + RBV N=781 % | Placebo + Peg-IFN-alfa + RBV N=397 % |
|---|---|---|---|
|
|||
| Chemistry | |||
| Alkaline phosphatase† | |||
| Grade 1 | > 1.25 to ≤ 2.50 × ULN‡ | 3 | 1 |
| Grade 2 | > 2.50 to ≤ 5.00 × ULN | < 1 | 0 |
| Hyperbilirubinemia | |||
| Grade 1 | > 1.1 to ≤ 1.5 × ULN | 27 | 15 |
| Grade 2 | > 1.5 to ≤ 2.5 × ULN | 18 | 9 |
| Grade 3 | > 2.5 to ≤ 5.0 × ULN | 4 | 2 |
| Grade 4 | > 5.0 × ULN | < 1 | 0 |
Elevations in bilirubin were predominately mild to moderate (Grade 1 or 2) in severity, and included elevation of both direct and indirect bilirubin. Elevations in bilirubin occurred early after treatment initiation, peaking by study Week 2, and were rapidly reversible upon cessation of OLYSIO. Bilirubin elevations were generally not associated with elevations in liver transaminases. The frequency of elevated bilirubin was higher in subjects with higher simeprevir exposures.
6.2 Postmarketing Experience
The following adverse reactions have been reported during post approval use of OLYSIO. Because postmarketing reactions are reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship between drug exposure and these adverse reactions.
7. Drug Interactions
7.1 Potential for OLYSIO to Affect Other Drugs
Simeprevir mildly inhibits CYP1A2 activity and intestinal CYP3A4 activity, but does not affect hepatic CYP3A4 activity. Coadministration of OLYSIO with drugs that are primarily metabolized by CYP3A4 may result in increased plasma concentrations of such drugs (see Table 8).
Simeprevir inhibits OATP1B1/3, P-glycoprotein (P-gp) and BCRP transporters, and does not inhibit OCT2 in vitro. Coadministration of OLYSIO with drugs that are substrates for OATP1B1/3, and P-gp and BCRP transport may result in increased plasma concentrations of such drugs (see Table 8).
Fluctuations in INR values may occur in patients receiving warfarin concomitant with HCV treatment, including treatment with OLYSIO. Close monitoring of INR values is recommended during treatment and post-treatment follow-up.
7.2 Potential for Other Drugs to Affect OLYSIO
The primary enzyme involved in the biotransformation of simeprevir is CYP3A [see Clinical Pharmacology (12.3)]. Clinically relevant effects of other drugs on simeprevir pharmacokinetics via CYP3A may occur. Coadministration of OLYSIO with moderate or strong inhibitors of CYP3A may significantly increase the plasma exposure of simeprevir. Coadministration with moderate or strong inducers of CYP3A may significantly reduce the plasma exposure of simeprevir and lead to loss of efficacy (see Table 8). Therefore, coadministration of OLYSIO with substances that are moderate or strong inducers or inhibitors of CYP3A is not recommended [see Warnings and Precautions (5.8) and Clinical Pharmacology (12.3)].
7.3 Established and Other Potentially Significant Drug Interactions
Table 8 shows the established and other potentially significant drug interactions based on which alterations in dose or regimen of OLYSIO and/or coadministered drug may be recommended. Drugs that are not recommended for coadministration with OLYSIO are also included in Table 8. For information regarding the magnitude of interaction, see Tables 9 and 10 [see Clinical Pharmacology (12.3)].
| Concomitant Drug Class Drug Name | Effect on Concentration of Simeprevir or Concomitant Drug | Clinical Comment |
|---|---|---|
| The direction of the arrow (↑ = increase, ↓ = decrease, ↔ = no change) indicates the direction of the change in PK. | ||
|
||
| Antiarrhythmics | ||
| Amiodarone | Effect on amiodarone, simeprevir, and sofosbuvir concentrations unknown | Coadministration of amiodarone with OLYSIO in combination with sofosbuvir is not recommended because it may result in serious symptomatic bradycardia. If coadministration is required, cardiac monitoring is recommended [see Warnings and Precautions (5.2), Adverse Reactions (6.2)]. |
| ↑ amiodarone | Caution is warranted and therapeutic drug monitoring of amiodarone, if available, is recommended for concomitant use of amiodarone with an OLYSIO-containing regimen that does not contain sofosbuvir. | |
| Digoxin* | ↑ digoxin | Routine therapeutic drug monitoring of digoxin concentrations is recommended. |
| Oral administration
Disopyramide, Flecainide, Mexiletine, Propafenone, Quinidine | ↑ antiarrhythmics | Therapeutic drug monitoring for these antiarrhythmics, if available, is recommended when coadministered with OLYSIO. |
| Anticonvulsants | ||
| Carbamazepine, Oxcarbazepine, Phenobarbital, Phenytoin | ↓ simeprevir | Coadministration is not recommended. |
| Anti-infectives | ||
| Antibiotics (systemic administration):
Erythromycin* | ↑ simeprevir ↑ erythromycin | Coadministration is not recommended. |
| Antibiotics (systemic administration):
Clarithromycin, Telithromycin | ↑ simeprevir | Coadministration is not recommended. |
| Antifungals (systemic administration):
Itraconazole, Ketoconazole, Posaconazole | ↑ simeprevir | Coadministration is not recommended. |
| Antifungals (systemic administration):
Fluconazole, Voriconazole | ↑ simeprevir | Coadministration is not recommended. |
| Antimycobacterials:
Rifampin*†, Rifabutin, Rifapentine | ↓ simeprevir ↔ rifampin, rifabutin, rifapentine | Coadministration is not recommended. |
| Calcium Channel Blockers (oral administration) | ||
| Amlodipine, Diltiazem, Felodipine, Nicardipine, Nifedipine, Nisoldipine, Verapamil | ↑ calcium channel blockers | Clinical monitoring of patients is recommended when OLYSIO is coadministered with calcium channel blockers. |
| Corticosteroids | ||
| Systemic
Dexamethasone | ↓ simeprevir | Coadministration is not recommended. |
| Gastrointestinal Products | ||
| Propulsive:
Cisapride | ↑ cisapride | Coadministration is not recommended. |
| HCV Products | ||
| Antiviral:
Ledipasvir‡ | ↑ ledipasvir ↑ simeprevir | Coadministration of OLYSIO with products containing ledipasvir is not recommended. |
| Herbal Products | ||
| Milk thistle (Silybum marianum) | ↑ simeprevir | Coadministration is not recommended. |
| St. John's wort (Hypericum perforatum) | ↓ simeprevir | Coadministration of OLYSIO with products containing St. John's wort is not recommended. |
| HIV Products | ||
| Cobicistat-containing products | ↑ simeprevir | Coadministration is not recommended. |
| Non-Nucleoside Reverse Transcriptase Inhibitors (NNRTIs):
Efavirenz* | ↓ simeprevir ↔ efavirenz | Coadministration is not recommended. |
| Other NNRTIs
Delavirdine Etravirine, Nevirapine |
↑ simeprevir ↓ simeprevir | Coadministration is not recommended. |
| Protease Inhibitors (PIs):
Darunavir/ritonavir*,§ | ↑ simeprevir ↑ darunavir | Coadministration is not recommended. |
| Protease Inhibitors (PIs):
Ritonavir*,¶ | ↑ simeprevir | Coadministration is not recommended. |
| Other ritonavir-boosted or unboosted HIV PIs (Atazanavir, Fosamprenavir, Lopinavir, Indinavir, Nelfinavir, Saquinavir, Tipranavir) | ↑ or ↓ simeprevir | Coadministration of OLYSIO with any HIV PI, with or without ritonavir is not recommended. |
| HMG CO-A Reductase Inhibitors | ||
| Atorvastatin, Rosuvastatin, Simvastatin* | ↑ statin | Coadministration of OLYSIO with statins is expected to increase statin concentrations, which is associated with increased risk of myopathy, including rhabdomyolysis. Use the lowest necessary statin dose, titrate the statin dose carefully, and monitor closely for statin-associated adverse reactions, such as myopathy or rhabdomyolysis. |
| Pitavastatin, Pravastatin, Lovastatin, Fluvastatin | ↑statin | |
| Immunosuppressants | ||
| Cyclosporine* | ↑ cyclosporine ↑ simeprevir# | Coadministration is not recommended. |
| Sirolimus | ↑ or ↓ sirolimus | Routine monitoring of blood concentrations of sirolimus is recommended. |
| Phosphodiesterase Type 5 (PDE-5) Inhibitors | ||
| Sildenafil, Tadalafil, Vardenafil | ↑ PDE-5 inhibitors | Dose adjustment of the PDE-5 inhibitor may be required when OLYSIO is coadministered with sildenafil or tadalafil administered chronically at doses used for the treatment of pulmonary arterial hypertension. Consider starting with the lowest dose of the PDE-5 inhibitor and increase as needed, with clinical monitoring as appropriate. No dose adjustment is required when OLYSIO is coadministered with doses of sildenafil, tadalafil or vardenafil indicated for the treatment of erectile dysfunction. |
| Sedatives/Anxiolytics | ||
| Midazolam* (oral administration) | ↑ midazolam | Caution is warranted when midazolam, which has a narrow therapeutic index, is coadministered with OLYSIO. |
| Triazolam (oral administration) | ↑ triazolam | Caution is warranted when triazolam, which has a narrow therapeutic index, is coadministered with OLYSIO. |
7.4 Drugs Without Clinically Significant Interactions with OLYSIO
In addition to the drugs included in Table 8, the interaction between OLYSIO and the following drugs were evaluated in clinical studies and no dose adjustments are needed for either drug [see Clinical Pharmacology (12.3)]: caffeine, daclatasvir, dextromethorphan, escitalopram, ethinyl estradiol/norethindrone, methadone, midazolam (intravenous administration), omeprazole, raltegravir, rilpivirine, sofosbuvir, tacrolimus, and tenofovir disoproxil fumarate.
No clinically relevant drug-drug interaction is expected when OLYSIO is coadministered with antacids, azithromycin, bedaquiline, corticosteroids (budesonide, fluticasone, methylprednisolone, and prednisone), dolutegravir, H2-receptor antagonists, the narcotic analgesics buprenorphine and naloxone, NRTIs (such as abacavir, didanosine, emtricitabine, lamivudine, stavudine, zidovudine), maraviroc, methylphenidate, and proton pump inhibitors.
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
If OLYSIO is administered with RBV, follow the recommendations for pregnancy testing and contraception within RBV's prescribing information. Refer to prescribing information for other drugs used in combination with OLYSIO for additional information on use in females and males of reproductive potential.
8.4 Pediatric Use
The safety and efficacy of OLYSIO in pediatric patients have not been established.
8.5 Geriatric Use
Clinical studies of OLYSIO did not include sufficient numbers of patients older than 65 years to determine whether they respond differently from younger patients. No dosage adjustment of OLYSIO is required in geriatric patients [see Clinical Pharmacology (12.3)].
8.6 Race
Patients of East Asian ancestry exhibit higher simeprevir plasma exposures, but no dosage adjustment is required based on race [see Adverse Reactions (6.1), Clinical Pharmacology (12.3) and Clinical Studies (14.3)].
8.7 Renal Impairment
No dosage adjustment of OLYSIO is required in patients with mild, moderate or severe renal impairment [see Clinical Pharmacology (12.3)]. The safety and efficacy of OLYSIO have not been studied in HCV-infected patients with severe renal impairment (creatinine clearance below 30 mL/min) or end-stage renal disease, including patients requiring dialysis. Simeprevir is highly protein-bound; therefore, dialysis is unlikely to result in significant removal of simeprevir [see Clinical Pharmacology (12.3)].
Refer to the prescribing information for the other antiviral drug(s) used in combination with OLYSIO regarding their use in patients with renal impairment.
8.8 Hepatic Impairment
No dosage adjustment of OLYSIO is required in patients with mild hepatic impairment (Child-Pugh A) [see Clinical Pharmacology (12.3)].
OLYSIO is not recommended for patients with moderate or severe hepatic impairment (Child-Pugh B or C). Simeprevir exposures are increased in patients with moderate or severe hepatic impairment (Child-Pugh B or C). In clinical trials of OLYSIO in combination with Peg-IFN-alfa and RBV, higher simeprevir exposures were associated with increased frequency of adverse reactions, including increased bilirubin, rash and photosensitivity. There have been postmarketing reports of hepatic decompensation, hepatic failure, and death in patients with advanced or decompensated cirrhosis receiving OLYSIO combination therapy [see Dosage and Administration (2.5), Warnings and Precautions (5.3), Adverse Reactions (6.1, 6.2), and Clinical Pharmacology (12.3)].
The safety and efficacy of OLYSIO have not been established in liver transplant patients.
See the Peg-IFN-alfa prescribing information regarding its contraindication in patients with hepatic decompensation.
10. Overdosage
Human experience of overdose with OLYSIO is limited. There is no specific antidote for overdose with OLYSIO. In the event of an overdose, the patient's clinical status should be observed and the usual supportive measures employed.
Simeprevir is highly protein-bound; therefore, dialysis is unlikely to result in significant removal of simeprevir [see Clinical Pharmacology (12.3)].
11. Olysio Description
OLYSIO (simeprevir) is an inhibitor of the HCV NS3/4A protease.
The chemical name for simeprevir is (2R,3aR,10Z,11aS,12aR,14aR)-N-(cyclopropylsulfonyl)-2-[[2-(4-isopropyl-1,3-thiazol-2-yl)-7-methoxy-8-methyl-4-quinolinyl]oxy]-5-methyl-4,14-dioxo-2,3,3a,4,5,6,7,8,9,11a,12,13,14,14a-tetradecahydrocyclopenta[c]cyclopropa[g][1,6]diazacyclotetradecine-12a(1H)-carboxamide. Its molecular formula is C38H47N5O7S2 and its molecular weight is 749.94. Simeprevir has the following structural formula:
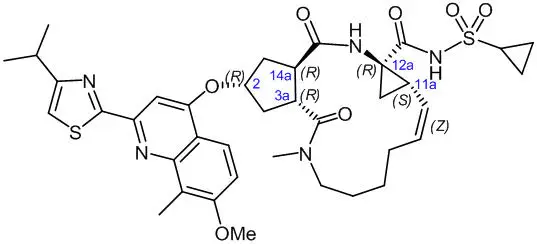
Simeprevir drug substance is a white to almost white powder. Simeprevir is practically insoluble in water over a wide pH range. It is practically insoluble in propylene glycol, very slightly soluble in ethanol, and slightly soluble in acetone. It is soluble in dichloromethane and freely soluble in some organic solvents (e.g., tetrahydrofuran and N,N-dimethylformamide).
OLYSIO (simeprevir) for oral administration is available as 150 mg strength hard gelatin capsules. Each capsule contains 154.4 mg of simeprevir sodium salt, which is equivalent to 150 mg of simeprevir. OLYSIO (simeprevir) capsules contain the following inactive ingredients: colloidal anhydrous silica, croscarmellose sodium, lactose monohydrate, magnesium stearate and sodium lauryl sulphate. The white capsule contains gelatin and titanium dioxide (E171) and is printed with ink containing iron oxide black (E172) and shellac (E904).
12. Olysio - Clinical Pharmacology
12.1 Mechanism of Action
Simeprevir is a direct-acting antiviral (DAA) agent against the hepatitis C virus [see Microbiology (12.4)].
12.3 Pharmacokinetics
The pharmacokinetic properties of simeprevir have been evaluated in healthy adult subjects and in adult HCV-infected subjects. Plasma Cmax and AUC increased more than dose-proportionally after multiple doses between 75 mg and 200 mg once daily, with accumulation occurring following repeated dosing. Steady-state was reached after 7 days of once-daily dosing. Plasma exposure (AUC) of simeprevir in HCV-infected subjects was about 2- to 3-fold higher compared to that observed in HCV-uninfected subjects. Plasma Cmax and AUC of simeprevir were similar during coadministration of simeprevir with Peg-IFN-alfa and RBV compared with administration of simeprevir alone. In Phase 3 trials with Peg-IFN-alfa and RBV in HCV-infected subjects, the geometric mean steady-state pre-dose plasma concentration was 1009 ng/mL (geometric coefficient of variation [gCV] = 162%) and the geometric mean steady-state AUC24 was 39140 ng.h/mL (gCV = 98%).
Drug Interactions
[See also Warnings and Precautions (5.8) and Drug Interactions (7)]
In vitro studies indicated that simeprevir is a substrate and mild inhibitor of CYP3A. Simeprevir does not affect CYP2C9, CYP2C19 or CYP2D6 in vivo. Simeprevir does not induce CYP1A2 or CYP3A4 in vitro. In vivo, simeprevir mildly inhibits the CYP1A2 activity and intestinal CYP3A4 activity, while it does not affect hepatic CYP3A4 activity. Simeprevir is not a clinically relevant inhibitor of cathepsin A enzyme activity.
In vitro, simeprevir is a substrate for P-gp, MRP2, BCRP, OATP1B1/3 and OATP2B1; simeprevir inhibits the uptake transporters OATP1B1/3 and NTCP and the efflux transporters P-gp/MDR1, MRP2, BCRP and BSEP and does not inhibit OCT2. The inhibitory effects of simeprevir on the bilirubin transporters OATP1B1/3 and MRP2 likely contribute to clinical observations of elevated bilirubin [see Adverse Reactions (6.1)].
Simeprevir is transported into the liver by OATP1B1/3 where it undergoes metabolism by CYP3A. Based on results from in vivo studies, coadministration of OLYSIO with moderate or strong inhibitors of CYP3A may significantly increase the plasma exposure of simeprevir and coadministration with moderate or strong inducers of CYP3A may significantly reduce the plasma exposure of simeprevir, which may lead to loss of efficacy.
Drug interaction studies were performed in healthy adults with simeprevir (at the recommended dose of 150 mg once daily unless otherwise noted) and drugs likely to be coadministered or drugs commonly used as probes for pharmacokinetic interactions. The effects of coadministration of other drugs on the Cmax, AUC, and Cmin values of simeprevir are summarized in Table 9 (effect of other drugs on OLYSIO). The effect of coadministration of OLYSIO on the Cmax, AUC, and Cmin values of other drugs are summarized in Table 10 (effect of OLYSIO on other drugs). For information regarding clinical recommendations, see Drug Interactions (7).
| Coadministered Drug | Dose (mg) and Schedule | N | Effect on PK* | LS Mean Ratio (90% CI) of Simeprevir PK Parameters with/without Drug | |||
|---|---|---|---|---|---|---|---|
| Drug | Simeprevir | Cmax | AUC | Cmin | |||
| CI = Confidence Interval; N = number of subjects with data; NA = not available; PK = pharmacokinetics; LS = least square; q.d. = once daily; b.i.d. = twice daily; t.i.d. = three times a day | |||||||
|
|||||||
| Cyclosporine† | individualized dose‡ | 150 mg q.d. for 14 days | 10 | ↑ | 4.53 (3.05–6.74) | 5.68 (3.58–9.00) | NA |
| Erythromycin | 500 mg t.i.d. for 7 days | 150 mg q.d. for 7 days | 24 | ↑ | 4.53 (3.91–5.25) | 7.47 (6.41–8.70) | 12.74 (10.19–15.93) |
| Escitalopram | 10 mg q.d. for 7 days | 150 mg q.d. for 7 days | 18 | ↓ | 0.80 (0.71–0.89) | 0.75 (0.68–0.83) | 0.68 (0.59–0.79) |
| Rifampin | 600 mg q.d. for 7 days | 200 mg q.d. for 7 days | 18 | ↓ | 1.31 (1.03–1.66) | 0.52 (0.41–0.67) | 0.08 (0.06–0.11) |
| Tacrolimus† | individualized dose‡ | 150 mg q.d. for 14 days | 25 | ↑ | 1.85 (1.40–2.46) | 1.90 (1.37–2.63) | NA |
| Anti-HCV Drug | |||||||
| Daclatasvir | 60 mg q.d. for 7 days | 150 mg q.d. for 7 days | 24 | ↑ | 1.39 (1.27–1.52) | 1.44 (1.32–1.56) | 1.49 (1.33–1.67) |
| Ledipasvir§ | 90 mg q.d. for 14 days | 150 mg q.d. for 14 days | 20 | ↑ | 2.34 (1.95–2.81) | 3.05 (2.34–3.84) | 4.69 (3.40–6.47) |
| Sofosbuvir¶ | 400 mg q.d. | 150 mg q.d. | 21 | ↔ | 0.96 (0.71–1.30) | 0.94 (0.67–1.33) | NA |
| Anti-HIV Drugs | |||||||
| Darunavir/Ritonavir# | 800/100 mg q.d. for 7 days | 50 mg and 150 mg q.d. for 7 days | 25 | ↑ | 1.79 (1.55–2.06) | 2.59 (2.15–3.11) | 4.58 (3.54–5.92) |
| Efavirenz | 600 mg q.d. for 14 days | 150 mg q.d. for 14 days | 23 | ↓ | 0.49 (0.44–0.54) | 0.29 (0.26–0.33) | 0.09 (0.08–0.12) |
| Raltegravir | 400 mg b.i.d. for 7 days | 150 mg q.d. for 7 days | 24 | ↔ | 0.93 (0.85–1.02) | 0.89 (0.81–0.98) | 0.86 (0.75–0.98) |
| Rilpivirine | 25 mg q.d. for 11 days | 150 mg q.d. for 11 days | 21 | ↔ | 1.10 (0.97–1.26) | 1.06 (0.94–1.19) | 0.96 (0.83–1.11) |
| Ritonavir | 100 mg b.i.d. for 15 days | 200 mg q.d. for 7 days | 12 | ↑ | 4.70 (3.84–5.76) | 7.18 (5.63–9.15) | 14.35 (10.29–20.01) |
| Tenofovir disoproxil fumarate | 300 mg q.d. for 7 days | 150 mg q.d. for 7 days | 24 | ↓ | 0.85 (0.73–0.99) | 0.86 (0.76–0.98) | 0.93 (0.78–1.11) |
| Coadministered Drug | Dose (mg) and Schedule | N | Effect on PK* | LS Mean Ratio (90% CI) of Coadministered Drug PK Parameters with/without OLYSIO | |||
|---|---|---|---|---|---|---|---|
| Drug | Simeprevir | Cmax | AUC | Cmin | |||
| CI = Confidence Interval; i.v.= intravenous; N = number of subjects with data; NA = not available; PK = pharmacokinetics; LS = least square; q.d. = once daily; b.i.d. = twice daily; t.i.d. = three times a day | |||||||
|
|||||||
| Atorvastatin | 40 mg single dose | 150 mg q.d. for 10 days | 18 | ↑ | 1.70 (1.42–2.04) | 2.12 (1.72–2.62) | NA |
| 2-hydroxy-atorvastatin | ↑ | 1.98 (1.70–2.31) | 2.29 (2.08–2.52) | NA | |||
| Caffeine | 150 mg | 150 mg q.d. for 11 days | 16 | ↑ | 1.12 (1.06–1.19) | 1.26 (1.21–1.32) | NA |
| Cyclosporine | 100 mg single dose | 150 mg q.d. for 7 days | 14 | ↑ | 1.16 (1.07–1.26) | 1.19 (1.13–1.26) | NA |
| Dextromethorphan | 30 mg | 150 mg q.d. for 11 days | 16 | ↑ | 1.21 (0.93–1.57) | 1.08 (0.87–1.35) | NA |
| Dextrorphan | ↔ | 1.03 (0.93–1.15) | 1.09 (1.03–1.15) | NA | |||
| Digoxin | 0.25 mg single dose | 150 mg q.d. for 7 days | 16 | ↑ | 1.31 (1.14–1.51) | 1.39 (1.16–1.67) | NA |
| Erythromycin | 500 mg t.i.d. for 7 days | 150 mg q.d. for 7 days | 24 | ↑ | 1.59 (1.23–2.05) | 1.90 (1.53–2.36) | 3.08 (2.54–3.73) |
| Escitalopram | 10 mg q.d. for 7 days | 150 mg q.d. for 7 days | 17 | ↔ | 1.03 (0.99–1.07) | 1.00 (0.97–1.03) | 1.00 (0.95–1.05) |
| Ethinyl estradiol (EE), coadministered with norethindrone (NE) | 0.035 mg q.d. EE + 1 mg q.d. NE for 21 days | 150 mg q.d. for 10 days | 18 | ↔ | 1.18 (1.09–1.27) | 1.12 (1.05–1.20) | 1.00 (0.89–1.13) |
| Midazolam (oral) | 0.075 mg/kg | 150 mg q.d. for 10 days | 16 | ↑ | 1.31 (1.19–1.45) | 1.45 (1.35–1.57) | NA |
| Midazolam (i.v.) | 0.025 mg/kg | 150 mg q.d. for 11 days | 16 | ↑ | 0.78 (0.52–1.17) | 1.10 (0.95–1.26) | NA |
| R(-) methadone† | 30–150 mg q.d., individualized dose | 150 mg q.d. for 7 days | 12 | ↔ | 1.03 (0.97–1.09) | 0.99 (0.91–1.09) | 1.02 (0.93–1.12) |
| Norethindrone (NE), coadministered with EE | 0.035 mg q.d. EE + 1 mg q.d. NE for 21 days | 150 mg q.d. for 10 days | 18 | ↔ | 1.06 (0.99–1.14) | 1.15 (1.08–1.22) | 1.24 (1.13–1.35) |
| Omeprazole | 40 mg single dose | 150 mg q.d. for 11 days | 16 | ↑ | 1.14 (0.93–1.39) | 1.21 (1.00–1.46) | NA |
| Rifampin | 600 mg q.d. for 7 days | 200 mg q.d. for 7 days | 18 | ↔ | 0.92 (0.80–1.07) | 1.00 (0.93–1.08) | NA |
| 25-desacetyl-rifampin | 17 | ↑ | 1.08 (0.98–1.19) | 1.24 (1.13–1.36) | NA | ||
| Rosuvastatin | 10 mg single dose | 150 mg q.d. for 7 days | 16 | ↑ | 3.17 (2.57–3.91) | 2.81 (2.34–3.37) | NA |
| Simvastatin | 40 mg single dose | 150 mg q.d. for 10 days | 18 | ↑ | 1.46 (1.17–1.82) | 1.51 (1.32–1.73) | NA |
| Simvastatin acid | ↑ | 3.03 (2.49–3.69) | 1.88 (1.63–2.17) | NA | |||
| Tacrolimus | 2 mg single dose | 150 mg q.d. for 7 days | 14 | ↓ | 0.76 (0.65–0.90) | 0.83 (0.59–1.16) | NA |
| S-Warfarin | 10 mg single dose | 150 mg q.d. for 11 days | 16 | ↔ | 1.00 (0.94–1.06) | 1.04 (1.00–1.07) | NA |
| Anti-HCV Drug | |||||||
| Daclatasvir | 60 mg q.d. for 7 days | 150 mg q.d. for 7 days | 24 | ↑ | 1.50 (1.39–1.62) | 1.96 (1.84–2.10) | 2.68 (2.42–2.98) |
| Ledipasvir‡ | 90 mg q.d. for 14 days | 150 mg q.d. for 14 days | 20 | ↑ | 1.64 (1.45–1.86) | 1.75 (1.56–1.96) | 1.74 (1.55–1.97) |
| Sofosbuvir§ | 400 mg q.d. | 150 mg q.d. | 22 | ↑ | 1.91 (1.26–2.90) | 3.16 (2.25–4.44) | NA |
| GS-331007¶ | ↔ | 0.69 (0.52–0.93) | 1.09 (0.87–1.37) | NA | |||
| Anti-HIV Drugs | |||||||
| Darunavir# | 800 mg q.d. for 7 days | 50 mg q.d. for 7 days | 25 | ↑ | 1.04 (0.99–1.10) | 1.18 (1.11–1.25) | 1.31 (1.13–1.52) |
| Ritonavir# | 100 mg q.d. for 7 days | ↑ | 1.23 (1.14–1.32) | 1.32 (1.25–1.40) | 1.44 (1.30–1.61) |
||
| Efavirenz | 600 mg q.d. for 14 days | 150 mg q.d. for 14 days | 23 | ↔ | 0.97 (0.89–1.06) | 0.90 (0.85–0.95) | 0.87 (0.81–0.93) |
| Raltegravir | 400 mg b.i.d. for 7 days | 150 mg q.d. for 7 days | 24 | ↑ | 1.03 (0.78–1.36) | 1.08 (0.85–1.38) | 1.14 (0.97–1.36) |
| Rilpivirine | 25 mg q.d. for 11 days | 150 mg q.d. for 11 days | 23 | ↔ | 1.04 (0.95–1.13) | 1.12 (1.05–1.19) | 1.25 (1.16–1.35) |
| Tenofovir disoproxil fumarate | 300 mg q.d. for 7 days | 150 mg q.d. for 7 days | 24 | ↔ | 1.19 (1.10–1.30) | 1.18 (1.13–1.24) | 1.24 (1.15–1.33) |
12.4 Microbiology
Resistance in Clinical Studies
In a pooled analysis of subjects treated with 150 mg OLYSIO in combination with Peg-IFN-alfa and RBV who did not achieve SVR in the controlled Phase 2 and Phase 3 clinical trials (PILLAR, ASPIRE, QUEST 1 and QUEST 2, PROMISE), emerging virus with amino acid substitutions at NS3 positions Q80, S122, R155 and/or D168 were observed in 180 out of 197 (91%) subjects. Substitutions D168V and R155K alone or in combination with other substitutions at these positions emerged most frequently (Table 11). Most of these emerging substitutions have been shown to reduce susceptibility to simeprevir in cell culture replicon assays.
HCV genotype 1 subtype-specific patterns of simeprevir treatment-emergent amino acid substitutions were observed. HCV genotype 1a predominately had emerging R155K alone or in combination with amino acid substitutions at NS3 positions Q80, S122 and/or D168, while HCV genotype 1b had most often an emerging D168V substitution (Table 11). In HCV genotype 1a with a baseline Q80K amino acid polymorphism, an emerging R155K substitution was observed most frequently at failure.
| Emerging Amino Acid Substitutions in NS3 | Genotype 1a*
N=116 % (n) | Genotype 1b N=81 % (n) |
|---|---|---|
| Note: substitutions at NS3 position F43 and A156 were selected in cell culture and associated with reduced simeprevir activity in the replicon assay but were not observed at time of failure. | ||
|
||
| Any substitution at NS3 position F43, Q80, S122, R155, A156, or D168† | 95 (110) | 86 (70) |
| D168E | 15 (17) | 17 (14) |
| D168V | 10 (12) | 60 (49) |
| Q80R‡ | 4 (5) | 12 (10) |
| R155K | 77 (89) | 0 (0) |
| Q80X+D168X§ | 4 (5) | 14 (11) |
| R155X+D168X§ | 13 (15) | 4 (3) |
| Q80K‡, S122A/G/I/T‡, S122R, R155Q‡, D168A, D168F‡, D168H, D168T, I170T¶ | Less than 10% | Less than 10% |
The majority of HCV genotype 1-infected subjects treated with OLYSIO in combination with sofosbuvir (with or without RBV) for 12 or 24 weeks who did not achieve SVR due to virologic reasons and with sequencing data available had emerging NS3 amino acid substitutions at position 168 and/or R155K: 5 out of 6 subjects in COSMOS and 1 out of 3 subjects in OPTIMIST-1. The emerging NS3 amino acid substitutions were similar to those observed in subjects who did not achieve SVR following treatment with OLYSIO in combination with Peg-IFN-alfa and RBV. No emerging NS5B amino acid substitutions associated with sofosbuvir resistance were observed in subjects who did not achieve SVR following treatment of OLYSIO in combination with sofosbuvir (with or without RBV) for 12 or 24 weeks.
In the RESTORE trial in genotype 4-infected subjects, 30 out of 34 (88%) subjects who did not achieve SVR had emerging amino acid substitutions at NS3 positions Q80, T122, R155, A156 and/or D168 (mainly substitutions at position D168; 26 out of 34 [76%] subjects), similar to the emerging amino acid substitutions observed in genotype 1-infected subjects.
12.5 Pharmacogenomics
A genetic variant near the gene encoding interferon-lambda-3 (IL28B rs12979860, a C [cytosine] to T [thymine] substitution) is a strong predictor of response to Peg-IFN-alfa and RBV (PR). In the Phase 3 trials, IL28B genotype was a stratification factor.
Overall, SVR rates were lower in subjects with the CT and TT genotypes compared to those with the CC genotype (Tables 12 and 13). Among both treatment-naïve subjects and those who experienced previous treatment failures, subjects of all IL28B genotypes had the highest SVR rates with OLYSIO-containing regimens (Table 12).
| Trial (Population) | IL28B rs12979860 Genotype | OLYSIO + PR % (n/N) | Placebo + PR % (n/N) |
|---|---|---|---|
| SVR12: sustained virologic response 12 weeks after planned end of treatment (EOT). | |||
| QUEST 1 and QUEST 2 (treatment-naïve subjects) | C/C | 95 (144/152) | 80 (63/79) |
| C/T | 78 (228/292) | 41 (61/147) | |
| T/T | 61 (47/77) | 21 (8/38) | |
| PROMISE (prior relapsers) | C/C | 89 (55/62) | 53 (18/34) |
| C/T | 78 (131/167) | 34 (28/83) | |
| T/T | 65 (20/31) | 19 (3/16) | |
| Trial (Population) | IL28B rs12979860 Genotype | Treatment-Naïve Subjects % (n/N) | Prior Relapsers % (n/N) | Prior Partial Responders % (n/N) | Prior Null Responders % (n/N) |
|---|---|---|---|---|---|
| SVR12: sustained virologic response 12 weeks after planned EOT. | |||||
| C212 (HIV-1 co-infection) | C/C | 100 (15/15) | 100 (7/7) | 100 (1/1) | 80 (4/5) |
| C/T | 70 (19/27) | 100 (6/6) | 71 (5/7) | 53 (10/19) | |
| T/T | 80 (8/10) | 0 (0/2) | 50 (1/2) | 50 (2/4) | |
| RESTORE (HCV genotype 4) | C/C | 100 (7/7) | 100 (1/1) | - | - |
| C/T | 82 (14/17) | 82 (14/17) | 60 (3/5) | 41 (9/22) | |
| T/T | 80 (8/10) | 100 (4/4) | 60 (3/5) | 39 (7/18) | |
13. Nonclinical Toxicology
13.2 Animal Toxicology and/or Pharmacology
Cardiovascular toxicity consisting of acute endocardial and myocardial necrosis restricted to the left ventricular subendocardial area was seen in 2 out of 6 animals in a 2-week oral dog toxicity study at an exposure approximately 28 times the mean AUC in humans at the recommended daily dose of 150 mg. No cardiac findings were observed in a 6-month and a 9-month oral toxicity study at exposures, respectively, of 11 and 4 times the mean AUC in humans at the recommended daily dose of 150 mg.
If OLYSIO is administered with Peg-IFN-alfa and RBV, refer to the prescribing information for Peg-IFN-alfa and RBV for information on animal toxicology.
14. Clinical Studies
14.1 Overview of Clinical Trials
The efficacy of OLYSIO in combination with sofosbuvir in subjects with HCV genotype 1 infection was evaluated in one Phase 2 trial (COSMOS) in prior null responders and treatment-naïve subjects with compensated cirrhosis (Child-Pugh A) or without cirrhosis, and in two Phase 3 trials in subjects with compensated cirrhosis (Child-Pugh A) or without cirrhosis (OPTIMIST-2 and OPTIMIST-1, respectively) who were HCV treatment-naïve or treatment-experienced (following prior treatment with IFN [pegylated or non-pegylated], with or without RBV) (see Table 14). Efficacy data from OPTIMIST-2, which evaluated OLYSIO in combination with sofosbuvir in subjects with compensated cirrhosis, are not shown because subjects in this trial received a shorter than recommended duration of therapy.
| Trial | Population | Relevant Study Arms (Number of Subjects Treated) |
|---|---|---|
| GT: genotype; TN: treatment-naïve; TE: treatment-experienced. | ||
|
||
| COSMOS (open-label) | GT 1, TN or TE*, with compensated cirrhosis or without cirrhosis |
|
| OPTIMIST-1 (open-label) | GT 1, TN or TE†, without cirrhosis |
|
| OPTIMIST-2 (open-label) | GT 1, TN or TE†, with compensated cirrhosis |
|
The efficacy of OLYSIO in combination with Peg-IFN-alfa and RBV in patients with HCV genotype 1 infection was evaluated in three Phase 3 trials in treatment-naïve subjects (QUEST 1, QUEST 2 and TIGER), one Phase 3 trial in subjects who relapsed after prior interferon-based therapy (PROMISE), one Phase 2 trial in subjects who failed prior therapy with Peg-IFN and RBV (including prior relapsers, partial and null responders) (ASPIRE), and one Phase 3 trial in subjects with HCV genotype 1 and HIV-1 co-infection who were HCV treatment-naïve or failed previous HCV therapy with Peg-IFN and RBV (C212), as summarized in Table 15.
The efficacy of OLYSIO in combination with Peg-IFN-alfa and RBV in patients with HCV genotype 4 infection was evaluated in one Phase 3 trial in treatment-naïve subjects or subjects who failed previous therapy with Peg-IFN and RBV (RESTORE) (see Table 15).
| Trial | Population | Relevant Study Arms (Number of Subjects Treated) |
|---|---|---|
| GT: genotype; TN: treatment-naïve; TE: treatment-experienced, includes prior relapsers, partial responders and null responders following prior treatment with Peg-IFN and RBV. | ||
|
||
| QUEST-1 (double-blind) | GT 1, TN, with compensated cirrhosis or without cirrhosis |
|
| QUEST-2 (double-blind) | GT 1, TN, with compensated cirrhosis or without cirrhosis |
|
| TIGER (double-blind) | GT 1, TN, with compensated cirrhosis or without cirrhosis |
|
| PROMISE (double-blind) | GT 1, TE*, with compensated cirrhosis or without cirrhosis |
|
| ASPIRE (double-blind) | GT 1, TE, with compensated cirrhosis or without cirrhosis |
|
| C212 (open-label) | GT 1, TN or TE, with compensated cirrhosis or without cirrhosis, HCV/HIV-1 co-infected |
|
| RESTORE (open-label) | GT 4, TN or TE, with compensated cirrhosis or without cirrhosis |
|
Prior relapsers were subjects who had HCV RNA not detected at the end of prior IFN-based therapy and HCV RNA detected during follow-up; prior partial responders were subjects with prior on-treatment greater than or equal to 2 log10 reduction in HCV RNA from baseline at Week 12 and HCV RNA detected at the end of prior therapy with Peg-IFN and RBV; and null responders were subjects with prior on-treatment less than 2 log10 reduction in HCV RNA from baseline at Week 12 during prior therapy with Peg-IFN and RBV. These trials included subjects with compensated cirrhosis (Child-Pugh A) or without cirrhosis, HCV RNA of at least 10000 IU/mL, and liver histopathology consistent with chronic HCV infection. In subjects who were treatment-naïve and prior relapsers, the overall duration of treatment with Peg-IFN-alfa and RBV in the Phase 3 trials was response-guided. In these subjects, the planned total duration of HCV treatment was 24 weeks if the following on-treatment protocol-defined response-guided therapy (RGT) criteria were met: HCV RNA lower than 25 IU/mL (detected or not detected) at Week 4 AND HCV RNA not detected at Week 12. Plasma HCV RNA levels were measured using the Roche COBAS® TaqMan® HCV test (version 2.0), for use with the High Pure System (25 IU/mL lower limit of quantification and 15 IU/mL limit of detection). Treatment stopping rules for HCV therapy were used to ensure that subjects with inadequate on-treatment virologic response discontinued treatment in a timely manner. In the Phase 3 trial C212 in HCV/HIV-1 co-infected subjects, the total duration of treatment with Peg-IFN-alfa and RBV in treatment-naïve and prior relapser subjects with compensated cirrhosis was not response-guided; these subjects received a fixed total duration of HCV treatment of 48 weeks. The total duration of treatment with Peg-IFN-alfa and RBV in non-cirrhotic HCV/HIV-1 co-infected treatment-naïve or prior relapser subjects was response-guided using the same criteria.
14.2 OLYSIO in Combination with Sofosbuvir
Adult Subjects with HCV Genotype 1 Infection
The efficacy of OLYSIO (150 mg once daily) in combination with sofosbuvir (400 mg once daily) in HCV genotype 1-infected treatment-naïve or treatment-experienced subjects with compensated cirrhosis (Child-Pugh A) or without cirrhosis was demonstrated in one Phase 2 trial (COSMOS) and one Phase 3 trial (OPTIMIST-1).
The COSMOS trial was an open-label, randomized Phase 2 trial to investigate the efficacy and safety of 12 or 24 weeks of OLYSIO (150 mg once daily) in combination with sofosbuvir (400 mg once daily) without or with RBV in HCV genotype 1-infected prior null responders with METAVIR fibrosis score F0–F2, or treatment-naïve subjects and prior null responders with METAVIR fibrosis score F3–F4 and compensated liver disease.
Results from treatment arms containing RBV in addition to OLYSIO and sofosbuvir in the COSMOS trial are not shown because efficacy was similar with or without RBV, and thus addition of RBV to OLYSIO and sofosbuvir is not recommended. In this trial, 28 subjects received 12 weeks of OLYSIO in combination with sofosbuvir and 31 subjects received 24 weeks of OLYSIO in combination with sofosbuvir. These 59 subjects had a median age of 57 years (range 27 to 68 years; with 2% above 65 years); 53% were male; 76% were White, and 24% Black or African American; 46% had a BMI greater than or equal to 30 kg/m2; the median baseline HCV RNA level was 6.75 log10 IU/mL; 19%, 31% and 22% had METAVIR fibrosis scores F0–F1, F2 and F3, respectively, and 29% had METAVIR fibrosis score F4 (cirrhosis); 75% had HCV genotype 1a of which 41% carried Q80K at baseline, and 25% had HCV genotype 1b; 14% had IL28B CC genotype, 64% IL28B CT genotype, and 22% IL28B TT genotype; 75% were prior null responders to Peg-IFN-alfa and RBV, and 25% were treatment-naïve.
OPTIMIST-1 was an open-label, randomized Phase 3 trial in HCV genotype 1-infected subjects without cirrhosis who were treatment-naïve or treatment-experienced (including prior relapsers, non-responders and IFN-intolerant subjects). Subjects were randomized to treatment arms of different durations. One hundred fifty-five subjects received 12 weeks of OLYSIO with sofosbuvir. The 155 subjects without cirrhosis receiving 12 weeks of OLYSIO with sofosbuvir had a median age of 56 years (range 19 to 70 years; with 7% above 65 years); 53% were male; 78% were White, 20% Black or African American, and 16% Hispanic; 37% had a BMI ≥ 30 kg/m2; the median baseline HCV RNA level was 6.83 log10 IU/mL; 75% had HCV genotype 1a of which 40% had Q80K polymorphism at baseline, and 25% had HCV genotype 1b; 28% had IL28B CC genotype, 55% IL28B CT genotype, and 17% IL28B TT genotype; 74% were treatment-naïve and 26% were treatment-experienced.
In the COSMOS and OPTIMIST-1 trials, SVR12 was achieved in 170/176 (97%) subjects without cirrhosis treated with 12 weeks OLYSIO in combination with sofosbuvir, as shown in Table 16. In the COSMOS trial, 10/10 (100%) subjects with compensated cirrhosis (Child-Pugh A) who received 24 weeks of OLYSIO with sofosbuvir achieved SVR12.
| Response Rates | OLYSIO+sofosbuvir*
12 weeks N=176 % (n/N) |
|---|---|
| SVR12: sustained virologic response 12 weeks after actual (OPTIMIST-1) or planned (COSMOS) EOT. | |
|
|
| Overall SVR12 | 97 (170/176) |
| Outcome for subjects without SVR12 | |
| Viral relapse† | 3 (5/175) |
Among subjects without cirrhosis in OPTIMIST-1 who received 12 weeks of OLYSIO in combination with sofosbuvir, similar SVR12 rates were observed among subgroups, including: treatment-naïve and treatment-experienced subjects (112/115 [97%] and 38/40 [95%] respectively), subjects with HCV genotype 1a with and without NS3 Q80K polymorphism (44/46 [96%] and 68/70 [97%], respectively), genotype 1b (38/39 [97%]), and subjects with IL28B CC and non-CC genotypes (43/43 [100%] and 107/112 [96%], respectively).
14.3 OLYSIO in Combination with Peg-IFN-alfa and RBV
Treatment-Naïve Adult Subjects with HCV Genotype 1 Infection
The efficacy of OLYSIO in treatment-naïve patients with HCV genotype 1 infection was demonstrated in two randomized, double-blind, placebo-controlled, 2-arm, multicenter, Phase 3 trials (QUEST 1 and QUEST 2). The designs of both trials were similar. All subjects received 12 weeks of once daily treatment with 150 mg OLYSIO or placebo, plus Peg-IFN-alfa-2a (QUEST 1 and QUEST 2) or Peg-IFN-alfa-2b (QUEST 2) and RBV, followed by 12 or 36 weeks of therapy with Peg-IFN-alfa and RBV in accordance with the on-treatment protocol-defined RGT criteria. Subjects in the control groups received 48 weeks of Peg-IFN-alfa-2a or -2b and RBV.
In the pooled analysis for QUEST 1 and QUEST 2, demographics and baseline characteristics were balanced between both trials and between the OLYSIO and placebo treatment groups. In the pooled analysis of trials (QUEST 1 and QUEST 2), the 785 enrolled subjects had a median age of 47 years (range: 18 to 73 years; with 2% above 65 years); 56% were male; 91% were White, 7% Black or African American, 1% Asian, and 17% Hispanic; 23% had a body mass index (BMI) greater than or equal to 30 kg/m2; 78% had baseline HCV RNA levels greater than 800000 IU/mL; 74% had METAVIR fibrosis score F0, F1 or F2, 16% METAVIR fibrosis score F3, and 10% METAVIR fibrosis score F4 (cirrhosis); 48% had HCV genotype 1a, and 51% HCV genotype 1b; 29% had IL28B CC genotype, 56% IL28B CT genotype, and 15% IL28B TT genotype; 17% of the overall population and 34% of the subjects with genotype 1a virus had the NS3 Q80K polymorphism at baseline. In QUEST 1, all subjects received Peg-IFN-alfa-2a; in QUEST 2, 69% of the subjects received Peg-IFN-alfa-2a and 31% received Peg-IFN-alfa-2b.
Table 17 shows the response rates in treatment-naïve adult subjects with HCV genotype 1 infection. In the OLYSIO treatment group, SVR12 rates were lower in subjects with genotype 1a virus with the NS3 Q80K polymorphism at baseline compared to subjects infected with genotype 1a virus without the Q80K polymorphism.
| Response Rate | OLYSIO + PR N=521 % (n/N) | Placebo + PR N=264 % (n/N) |
|---|---|---|
| OLYSIO: 150 mg OLYSIO for 12 weeks with Peg-IFN-alfa-2a or -2b and RBV for 24 or 48 weeks; Placebo: placebo for 12 weeks with Peg-IFN-alfa-2a or -2b and RBV for 48 weeks. SVR12: sustained virologic response 12 weeks after planned EOT. | ||
|
||
| Overall SVR12 (genotype 1a and 1b) | 80 (419/521) | 50 (132/264) |
| Genotype 1a | 75 (191/254) | 47 (62/131) |
| Without Q80K | 84 (138/165) | 43 (36/83) |
| With Q80K | 58 (49/84) | 52 (23/44) |
| Genotype 1b | 85 (228/267) | 53 (70/133) |
| Outcome for subjects without SVR12 | ||
| On-treatment failure* | 8 (42/521) | 33 (87/264) |
| Viral relapse† | 11 (51/470) | 23 (39/172) |
In the pooled analysis of QUEST 1 and QUEST 2, 88% (459/521) of OLYSIO-treated subjects were eligible for a total treatment duration of 24 weeks. In these subjects, the SVR12 rate was 88% (405/459).
Seventy-nine percent (79%; 404/509) of OLYSIO-treated subjects had HCV RNA not detected at Week 4 (RVR); in these subjects the SVR12 rate was 90% (362/404).
SVR12 rates were higher for the OLYSIO treatment group compared to the placebo treatment group by sex, age, race, BMI, HCV genotype/subtype, baseline HCV RNA load (less than or equal to 800000 IU/mL, greater than 800000 IU/mL), METAVIR fibrosis score, and IL28B genotype. Table 18 shows the SVR rates by METAVIR fibrosis score.
| Subgroup | OLYSIO + PR % (n/N) | Placebo + PR % (n/N) |
|---|---|---|
| OLYSIO: 150 mg OLYSIO for 12 weeks with Peg-IFN-alfa-2a or -2b and RBV for 24 or 48 weeks; Placebo: placebo for 12 weeks with Peg-IFN-alfa-2a or -2b and RBV for 48 weeks. SVR12: sustained virologic response 12 weeks after planned EOT. | ||
| F0–2 | 84 (317/378) | 55 (106/192) |
| F3–4 | 68 (89/130) | 36 (26/72) |
SVR12 rates were higher for subjects receiving OLYSIO with Peg-IFN-alfa-2a or Peg-IFN-alfa-2b and RBV (88% and 78%, respectively) compared to subjects receiving placebo with Peg-IFN-alfa-2a or Peg-IFN-alfa-2b and RBV (62% and 42%, respectively) (QUEST 2).
Adult Subjects with HCV Genotype 1 Infection who Failed Prior Peg-IFN-alfa and RBV Therapy
The PROMISE trial was a randomized, double-blind, placebo-controlled, 2-arm, multicenter, Phase 3 trial in subjects with HCV genotype 1 infection who relapsed after prior IFN-based therapy. All subjects received 12 weeks of once daily treatment with 150 mg OLYSIO or placebo, plus Peg-IFN-alfa-2a and RBV, followed by 12 or 36 weeks of therapy with Peg-IFN-alfa-2a and RBV in accordance with the protocol-defined RGT criteria. Subjects in the control group received 48 weeks of Peg-IFN-alfa-2a and RBV.
Demographics and baseline characteristics were balanced between the OLYSIO and placebo treatment groups. The 393 subjects enrolled in the PROMISE trial had a median age of 52 years (range: 20 to 71 years; with 3% above 65 years); 66% were male; 94% were White, 3% Black or African American, 2% Asian, and 7% Hispanic; 26% had a BMI greater than or equal to 30 kg/m2; 84% had baseline HCV RNA levels greater than 800000 IU/mL; 69% had METAVIR fibrosis score F0, F1 or F2, 15% METAVIR fibrosis score F3, and 15% METAVIR fibrosis score F4 (cirrhosis); 42% had HCV genotype 1a, and 58% HCV genotype 1b; 24% had IL28B CC genotype, 64% IL28B CT genotype, and 12% IL28B TT genotype; 13% of the overall population and 31% of the subjects with genotype 1a virus had the NS3 Q80K polymorphism at baseline. The prior IFN-based HCV therapy was Peg-IFN-alfa-2a/RBV (68%) or Peg-IFN-alfa-2b/RBV (27%).
Table 19 shows the response rates for the OLYSIO and placebo treatment groups in adult subjects with HCV genotype 1 infection who relapsed after prior interferon-based therapy. In the OLYSIO treatment group, SVR12 rates were lower in subjects infected with genotype 1a virus with the NS3 Q80K polymorphism at baseline compared to subjects infected with genotype 1a virus without the Q80K polymorphism.
| Response Rates | OLYSIO + PR N=260 % (n/N) | Placebo + PR N=133 % (n/N) |
|---|---|---|
| OLYSIO: 150 mg OLYSIO for 12 weeks with Peg-IFN-alfa-2a and RBV for 24 or 48 weeks; Placebo: placebo for 12 weeks with Peg-IFN-alfa-2a and RBV for 48 weeks. SVR12: sustained virologic response 12 weeks after planned EOT. | ||
|
||
| Overall SVR12 (genotype 1a and 1b) | 79 (206/260) | 37 (49/133) |
| Genotype 1a | 70 (78/111) | 28 (15/54) |
| Without Q80K | 78 (62/79) | 26 (9/34) |
| With Q80K | 47 (14/30) | 30 (6/20) |
| Genotype 1b | 86 (128/149) | 43 (34/79) |
| Outcome for subjects without SVR12 | ||
| On-treatment failure* | 3 (8/260) | 27 (36/133) |
| Viral relapse† | 18 (46/249) | 48 (45/93) |
In PROMISE, 93% (241/260) of OLYSIO-treated subjects were eligible for a total treatment duration of 24 weeks. In these subjects, the SVR12 rate was 83% (200/241).
Seventy-seven percent (77%; 200/259) of OLYSIO-treated subjects had HCV RNA not detected at Week 4 (RVR); in these subjects the SVR12 rate was 87% (173/200).
SVR12 rates were higher for the OLYSIO treatment group compared to the placebo treatment group by sex, age, race, BMI, HCV genotype/subtype, baseline HCV RNA load (less than or equal to 800000 IU/mL, greater than 800000 IU/mL), prior HCV therapy, METAVIR fibrosis score, and IL28B genotype. Table 20 shows the SVR rates by METAVIR fibrosis score.
| Subgroup | OLYSIO + PR % (n/N) | Placebo + PR % (n/N) |
|---|---|---|
| OLYSIO: 150 mg OLYSIO for 12 weeks with Peg-IFN-alfa-2a and RBV for 24 or 48 weeks; Placebo: placebo for 12 weeks with Peg-IFN-alfa-2a and RBV for 48 weeks. SVR12: sustained virologic response 12 weeks after planned EOT. | ||
| F0–2 | 82 (137/167) | 41 (40/98) |
| F3–4 | 73 (61/83) | 24 (8/34) |
The ASPIRE trial was a randomized, double-blind, placebo-controlled, Phase 2 trial in subjects with HCV genotype 1 infection, who failed prior therapy with Peg-IFN-alfa and RBV (including prior relapsers, partial responders or null responders).
In this trial, 66 subjects received 12 weeks of 150 mg OLYSIO in combination with Peg-IFN-alfa-2a and RBV for 48 weeks, and 66 subjects received placebo in combination with Peg-IFN-alfa-2a and RBV for 48 weeks. These 132 subjects had a median age of 49 years (range: 20 to 66 years; with 1% above 65 years); 66% were male; 93% were White, 3% Black or African American, and 2% Asian; 27% had a BMI greater than or equal to 30 kg/m2; 85% had baseline HCV RNA levels greater than 800000 IU/mL; 64% had METAVIR fibrosis score F0, F1, or F2, 18% METAVIR fibrosis score F3, and 18% METAVIR fibrosis score F4 (cirrhosis); 43% had HCV genotype 1a, and 57% HCV genotype 1b; 17% had IL28B CC genotype, 67% IL28B CT genotype, and 16% IL28B TT genotype (information available for 93 subjects); 27% of the overall population and 23% of the subjects with genotype 1a virus had the NS3 Q80K polymorphism at baseline. Forty percent (40%) of subjects were prior relapsers, 35% prior partial responders, and 25% prior null responders following prior therapy with Peg-IFN-alfa and RBV. Demographics and baseline characteristics were balanced between the 12 weeks 150 mg OLYSIO and placebo treatment groups.
Table 21 shows the response rates for the 12 weeks of 150 mg OLYSIO and placebo treatment groups in prior relapsers, prior partial responders and prior null responders.
| Response Rates | OLYSIO + PR N=66 % (n/N) | Placebo + PR N=66 % (n/N) |
|---|---|---|
| 150 mg OLYSIO: 150 mg OLYSIO for 12 weeks with Peg-IFN-alfa-2a and RBV for 48 weeks; Placebo: placebo with Peg-IFN-alfa-2a and RBV for 48 weeks. | ||
| SVR24: sustained virologic response defined as undetectable HCV RNA 24 weeks after planned EOT. | ||
|
||
| SVR24 | ||
| Prior relapsers | 77 (20/26) | 37 (10/27) |
| Prior partial responders | 65 (15/23) | 9 (2/23) |
| Prior null responders | 53 (9/17) | 19 (3/16) |
| Outcome for subjects without SVR24 | ||
| On-treatment virologic failure* | ||
| Prior relapsers | 8 (2/26) | 22 (6/27) |
| Prior partial responders | 22 (5/23) | 78 (18/23) |
| Prior null responders | 35 (6/17) | 75 (12/16) |
| Viral relapse† | ||
| Prior relapsers | 13 (3/23) | 47 (9/19) |
| Prior partial responders | 6 (1/17) | 50 (2/4) |
| Prior null responders | 18 (2/11) | 25 (1/4) |
SVR24 rates were higher in the OLYSIO-treated subjects compared to subjects receiving placebo in combination with Peg-IFN-alfa and RBV, regardless of HCV geno/subtype, METAVIR fibrosis score, and IL28B genotype.
Subjects with HCV/HIV-1 Co-Infection
C212 was an open-label, single-arm Phase 3 trial in HIV-1 subjects co-infected with HCV genotype 1 who were treatment-naïve or failed prior HCV therapy with Peg-IFN-alfa and RBV (including prior relapsers, partial responders or null responders). Non-cirrhotic treatment-naïve subjects or prior relapsers received 12 weeks of once-daily treatment with 150 mg OLYSIO plus Peg-IFN-alfa-2a and RBV, followed by 12 or 36 weeks of therapy with Peg-IFN-alfa-2a and RBV in accordance with the protocol-defined RGT criteria. Prior non-responder subjects (partial and null response) and all cirrhotic subjects (METAVIR fibrosis score F4) received 36 weeks of Peg-IFN-alfa-2a and RBV after the initial 12 weeks of OLYSIO in combination with Peg-IFN-alfa-2a and RBV.
The 106 enrolled subjects in the C212 trial had a median age of 48 years (range: 27 to 67 years; with 2% above 65 years); 85% were male; 82% were White, 14% Black or African American, 1% Asian, and 6% Hispanic; 12% had a BMI greater than or equal to 30 kg/m2; 86% had baseline HCV RNA levels greater than 800,000 IU/mL; 68% had METAVIR fibrosis score F0, F1 or F2, 19% METAVIR fibrosis score F3, and 13% METAVIR fibrosis score F4; 82% had HCV genotype 1a, and 17% HCV genotype 1b; 28% of the overall population and 34% of the subjects with genotype 1a had Q80K polymorphism at baseline; 27% had IL28B CC genotype, 56% IL28B CT genotype, and 17% IL28B TT genotype; 50% (n=53) were HCV treatment-naïve subjects, 14% (n=15) prior relapsers, 9% (n=10) prior partial responders, and 26% (n=28) prior null responders. Eighty-eight percent (n=93) of the subjects were on highly active antiretroviral therapy (HAART), with nucleoside reverse transcriptase inhibitors and the integrase inhibitor raltegravir being the most commonly used HIV antiretroviral. HIV protease inhibitors and non-nucleoside reverse transcriptase inhibitors (except rilpivirine) were prohibited from use in this study.
The median baseline HIV-1 RNA levels and CD4+ cell count in subjects not on HAART were 4.18 log10 copies/mL (range: 1.3–4.9 log10 copies/mL) and 677 × 106 cells/L (range: 489–1076 × 106 cells/L), respectively. The median baseline CD4+ cell count in subjects on HAART was 561 × 106 cells/mL (range: 275–1407 × 106 cells/mL).
Table 22 shows the response rates in treatment-naïve, prior relapsers, prior partial responders and null responders.
| Response Rates | Treatment-Naïve Subjects N=53 % (n/N) | Prior Relapsers N=15 % (n/N) | Prior Partial Responders N=10 % (n/N) | Prior Null Responders N=28 % (n/N) |
|---|---|---|---|---|
| 150 mg OLYSIO for 12 weeks with Peg-IFN-alfa-2a and RBV for 24 or 48 weeks. | ||||
| SVR12: sustained virologic response 12 weeks after planned EOT. | ||||
|
||||
| Overall SVR12 (genotype 1a and 1b) | 79 (42/53) | 87 (13/15) | 70 (7/10) | 57 (16/28) |
| Genotype 1a | 77 (33/43) | 83 (10/12) | 67 (6/9) | 54 (13/24) |
| Genotype 1b | 90 (9/10) | 100 (3/3) | 100 (1/1) | 75 (3/4) |
| Outcome for subjects without SVR12 | ||||
| On-treatment failure* | 9 (5/53) | 0 (0/15) | 20 (2/10) | 39 (11/28) |
| Viral relapse† | 10 (5/48) | 13 (2/15) | 0 (0/7) | 12 (2/17) |
Eighty-nine percent (n=54/61) of the OLYSIO-treated treatment-naïve subjects and prior relapsers without cirrhosis were eligible for a total treatment duration of 24 weeks. In these subjects, the SVR12 rate was 87%.
Seventy-one percent (n=37/52), 93% (n=14/15), 80% (n=8/10) and 36% (n=10/28) of OLYSIO-treated treatment-naïve subjects, prior relapsers, prior partial responders and prior null responders had undetectable HCV RNA at week 4 (RVR). In these subjects the SVR12 rates were 89%, 93%, 75% and 90%, respectively.
Table 23 shows the SVR rates by METAVIR fibrosis scores.
| Subgroup | Treatment-Naïve Subjects % (n/N) | Prior Relapsers % (n/N) | Prior Partial Responders % (n/N) | Prior Null Responders % (n/N) |
|---|---|---|---|---|
| 150 mg OLYSIO for 12 weeks with Peg-IFN-alfa-2a and RBV for 24 or 48 weeks. | ||||
| SVR12: sustained virologic response 12 weeks after planned EOT. | ||||
| F0–2 | 89 (24/27) | 78 (7/9) | 50 (1/2) | 57 (4/7) |
| F3–4 | 57 (4/7) | 100 (2/2) | 67 (2/3) | 60 (6/10) |
Two subjects had HIV virologic failure defined as confirmed HIV-1 RNA at least 200 copies/mL after previous less than 50 copies/mL; these failures occurred 36 and 48 weeks after end of OLYSIO treatment.
Adult Subjects with HCV Genotype 4 Infection
RESTORE was an open-label, single-arm Phase 3 trial in subjects with HCV genotype 4 infection who were treatment-naïve or failed prior therapy with Peg-IFN-alfa and RBV (including prior relapsers, partial responders or null responders). Treatment-naïve subjects or prior relapsers received once-daily treatment with 150 mg OLYSIO plus Peg-IFN-alfa-2a and RBV for 12 weeks, followed by 12 or 36 weeks of therapy with Peg-IFN-alfa-2a and RBV in accordance with the protocol-defined RGT criteria. Prior non-responder subjects (partial and null response) received once-daily treatment with 150 mg OLYSIO plus Peg-IFN-alfa-2a and RBV for 12 weeks, followed by 36 weeks of Peg-IFN-alfa-2a and RBV.
The 107 enrolled subjects in the RESTORE trial with HCV genotype 4 had a median age of 49 years (range: 27 to 69 years; with 5% above 65 years); 79% were male; 72% were White, 28% Black or African American, and 7% Hispanic; 14% had a BMI greater than or equal to 30 kg/m2; 60% had baseline HCV RNA levels greater than 800,000 IU/mL; 57% had METAVIR fibrosis score F0, F1 or F2, 14% METAVIR fibrosis score F3, and 29% METAVIR fibrosis score F4; 42% had HCV genotype 4a, and 24% had HCV genotype 4d; 8% had IL28B CC genotype, 58% IL28B CT genotype, and 35% IL28B TT genotype; 33% (n=35) were treatment-naïve HCV subjects, 21% (n=22) prior relapsers, 9% (n=10) prior partial responders, and 37% (n=40) prior null responders.
Table 24 shows the response rates in treatment-naïve, prior relapsers, prior partial responders and null responders. Table 25 shows the SVR rates by METAVIR fibrosis scores.
| Response Rates | Treatment-Naïve Subjects N=35 % (n/N) | Prior Relapsers N=22 % (n/N) | Prior Partial Responders N=10 % (n/N) | Prior Null Responders N=40 % (n/N) |
|---|---|---|---|---|
| 150 mg OLYSIO for 12 weeks with Peg-IFN-alfa-2a and RBV for 24 or 48 weeks. | ||||
| SVR12: sustained virologic response 12 weeks after planned EOT. | ||||
|
||||
| Overall SVR12 | 83 (29/35) | 86 (19/22) | 60 (6/10) | 40 (16/40) |
| Outcome for subjects without SVR12 | ||||
| On-treatment failure* | 9 (3/35) | 9 (2/22) | 20 (2/10) | 45 (18/40) |
| Viral relapse† | 9 (3/35) | 5 (1/22) | 20 (2/10) | 15 (6/40) |
| Subgroup | Treatment-Naïve Subjects % (n/N) | Prior Relapsers % (n/N) | Prior Partial Responders % (n/N) | Prior Null Responders % (n/N) |
|---|---|---|---|---|
| 150 mg OLYSIO for 12 weeks with Peg-IFN-alfa-2a and RBV for 24 or 48 weeks. | ||||
| SVR12: sustained virologic response 12 weeks after planned EOT. | ||||
| F0–2 | 85 (22/26) | 91 (10/11) | 100 (5/5) | 47 (8/17) |
| F3–4 | 78 (7/9) | 82 (9/11) | 20 (1/5) | 35 (7/20) |
16. How is Olysio supplied
OLYSIO 150 mg capsules are white, marked with "TMC435 150" in black ink. The capsules are packaged into a bottle containing 28 capsules (NDC 59676-225-28).
17. Patient Counseling Information
Advise patients to read the FDA-approved patient labeling (Patient Information).
| This Patient Information has been approved by the U.S. Food and Drug Administration. | Revised February 2017 | |||
| PATIENT INFORMATION OLYSIO® (oh li see oh) (simeprevir) capsules |
||||
| Read this Patient Information before you start taking OLYSIO and each time you get a refill. There may be new information. This information does not take the place of talking with your healthcare provider about your medical condition or your treatment. | ||||
| Important: You should not take OLYSIO alone. OLYSIO should be used together with other antiviral medicines to treat chronic hepatitis C virus infection. When taking OLYSIO in combination with peginterferon alfa and ribavirin you should also read those Medication Guides. When taking OLYSIO in combination with sofosbuvir, you should also read its Patient Information leaflet. | ||||
| What is the most important information I should know about OLYSIO?
OLYSIO can cause serious side effects, including: Hepatitis B virus reactivation: Before starting treatment with OLYSIO, your healthcare provider will do blood tests to check for hepatitis B virus infection. If you have ever had hepatitis B virus infection, the hepatitis B virus could become active again during or after treatment of hepatitis C virus with OLYSIO. Hepatitis B virus becoming active again (called reactivation) may cause serious liver problems including liver failure and death. Your healthcare provider will monitor you if you are at risk for hepatitis B virus reactivation during treatment and after you stop taking OLYSIO. OLYSIO combination treatment with sofosbuvir (Sovaldi®) may result in slowing of the heart rate (pulse) along with other symptoms when taken with amiodarone (Cordarone®, Nexterone®, Pacerone®), a medicine used to treat certain heart problems.
|
||||
|
|
|||
|
OLYSIO may cause severe liver problems in some people. These severe liver problems may lead to liver failure or death.
|
||||
|
|
|||
|
OLYSIO combination treatment may cause rashes and skin reactions to sunlight. These rashes and skin reactions to sunlight can be severe and you may need to be treated in a hospital. Rashes and skin reactions to sunlight are most common during the first 4 weeks of treatment, but can happen at any time during combination treatment with OLYSIO.
|
||||
|
|
|||
| For more information about side effects, see the section "What are the possible side effects of OLYSIO?" | ||||
|---|---|---|---|---|
What is OLYSIO?
It is not known if OLYSIO is safe and effective in children under 18 years of age. |
||||
| What should I tell my healthcare provider before taking OLYSIO?
Before taking OLYSIO, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. Some medicines may interact with OLYSIO. This can cause you to have too much or not enough OLYSIO or other medicines in your body, which may affect the way OLYSIO or your other medicines work, or may cause side effects. Keep a list of your medicines and show it to your healthcare provider and pharmacist.
|
||||
How should I take OLYSIO?
|
||||
| What are the possible side effects of OLYSIO?
OLYSIO can cause serious side effects, including:
|
||||
|
The most common side effects of OLYSIO when used in combination with sofosbuvir include: |
||||
|
|
|
||
|
The most common side effects of OLYSIO when used in combination with peginterferon alfa and ribavirin include: |
||||
|
|
|
||
|
Tell your healthcare provider if you have any side effect that bothers you or that does not go away. These are not all of the possible side effects of OLYSIO. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
How should I store OLYSIO?
Keep OLYSIO and all medicines out of the reach of children. |
||||
| General information about the safe and effective use of OLYSIO
Medicines are sometimes prescribed for purposes other than those listed in a Patient Information leaflet. Do not use OLYSIO for a condition for which it was not prescribed. Do not give your OLYSIO to other people, even if they have the same symptoms that you have. It may harm them. If you would like more information about OLYSIO, talk with your pharmacist or healthcare provider. You can ask your pharmacist or healthcare provider for information about OLYSIO that is written for health professionals. For more information about OLYSIO, go to www.OLYSIO.com or call 1-800-526-7736. |
||||
| What are the ingredients in OLYSIO?
Active ingredient: simeprevir Inactive ingredients: colloidal anhydrous silica, croscarmellose sodium, lactose monohydrate, magnesium stearate, sodium lauryl sulphate. The white capsule contains gelatin and titanium dioxide (E171) and is printed with ink containing iron oxide black (E172) and shellac (E904). Product of Belgium Manufactured by: Janssen-Cilag SpA, Latina, Italy Manufactured for: Janssen Therapeutics, Division of Janssen Products, LP, Titusville NJ 08560 Licensed from Medivir AB OLYSIO® is a registered trademark of Johnson & Johnson © Janssen Products, LP 2017 |
||||
| OLYSIO
simeprevir capsule |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Janssen Products LP (804684207) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Pharmaceutica NV | 374747970 | API MANUFACTURE(59676-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Janssen Cilag SpA | 542797928 | MANUFACTURE(59676-225) , ANALYSIS(59676-225) , PACK(59676-225) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc. | 053217022 | LABEL(59676-225) | |





