Drug Detail:Orkambi (Ivacaftor and lumacaftor [ eye-va-kaf-tor-and-loo-ma-kaf-tor ])
Drug Class: CFTR combinations
Highlights of Prescribing Information
ORKAMBI® (lumacaftor and ivacaftor) tablets, for oral use
ORKAMBI® (lumacaftor and ivacaftor) oral granules
Initial U.S. Approval: 2015
Recent Major Changes
| Indications and Usage (1) | 09/2022 |
| Dosage and Administration (2) | 09/2022 |
| Warnings and Precautions, Hypersensitivity Reactions, Including Anaphylaxis (5.3) | 08/2023 |
Indications and Usage for Orkambi
ORKAMBI is a combination of ivacaftor, a cystic fibrosis transmembrane conductance regulator (CFTR) potentiator, and lumacaftor, indicated for the treatment of cystic fibrosis (CF) in patients aged 1 year and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of the F508del mutation on both alleles of the CFTR gene. (1)
Limitations of Use:
The efficacy and safety of ORKAMBI have not been established in patients with CF other than those homozygous for the F508del mutation. (1)
Orkambi Dosage and Administration
| Age Group | Weight | Dose | Administration |
|---|---|---|---|
| 1 through 2 years | 7 kg to < 9 kg | 1 packet of lumacaftor 75 mg/ivacaftor 94 mg granules | Mixed with one teaspoon (5 mL) of soft food or liquid and administered orally every 12 hours with fat-containing food |
| 9 kg to < 14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg granules | ||
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg granules | ||
| 2 through 5 years | <14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg granules | |
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg granules | ||
| 6 through 11 years | - | 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg (lumacaftor 200 mg/ivacaftor 250 mg per dose) | Taken orally every 12 hours with fat-containing food |
| 12 years and older | - | 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg (lumacaftor 400 mg/ivacaftor 250 mg per dose) |
- Reduce dosage in patients with moderate or severe hepatic impairment. (2.2, 8.6, 12.3)
- When initiating ORKAMBI in patients taking strong CYP3A inhibitors, reduce ORKAMBI dosage for the first week of treatment. (2.3, 7.1, 12.3)
Dosage Forms and Strengths
- Tablets: lumacaftor 100 mg and ivacaftor 125 mg; lumacaftor 200 mg and ivacaftor 125 mg. (3)
- Oral granules: Unit-dose packets of lumacaftor 75 mg and ivacaftor 94 mg; lumacaftor 100 mg and ivacaftor 125 mg; lumacaftor 150 mg and ivacaftor 188 mg. (3)
Contraindications
- None. (4)
Warnings and Precautions
- Use in patients with advanced liver disease: ORKAMBI should be used with caution in these patients and only if the benefits are expected to outweigh the risks. If ORKAMBI is used in these patients, they should be closely monitored after the initiation of treatment and the dose should be reduced. Liver function decompensation, including liver failure leading to death, has been reported in CF patients with pre-existing cirrhosis with portal hypertension. (2.2, 5.1, 6.1)
- Liver-related events: Elevated transaminases (ALT/AST) have been observed in some cases associated with elevated bilirubin. Measure serum transaminases and bilirubin before initiating ORKAMBI, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of ALT, AST, or bilirubin elevations, more frequent monitoring should be considered. Interrupt dosing in patients with ALT or AST >5 × upper limit of normal (ULN), or ALT or AST >3 × ULN with bilirubin >2 × ULN. Following resolution, consider the benefits and risks of resuming dosing. (5.2, 6.1)
- Hypersensitivity reactions: Angioedema and anaphylaxis have been reported with ORKAMBI in the postmarketing setting. Initiate appropriate therapy in the event of a hypersensitivity reaction. (5.3)
- Respiratory events: Chest discomfort, dyspnea, and respiration abnormal were observed more commonly during initiation of ORKAMBI. Clinical experience in patients with percent predicted FEV1 (ppFEV1) <40 is limited, and additional monitoring of these patients is recommended during initiation of therapy. (5.4, 6.1)
- Blood pressure: Increased blood pressure has been observed in some patients. Periodically monitor blood pressure in all patients. (5.5, 6.1)
- Drug interactions: Use with sensitive CYP3A substrates or CYP3A substrates with a narrow therapeutic index may decrease systemic exposure of the medicinal products and co-administration is not recommended. Hormonal contraceptives should not be relied upon as an effective method of contraception and their use is associated with increased menstruation-related adverse reactions. Use with strong CYP3A inducers may diminish exposure of ivacaftor, which may diminish its effectiveness; therefore, co-administration is not recommended. (5.6, 6.1, 7, 12.3)
- Cataracts: Non-congenital lens opacities/cataracts have been reported in pediatric patients treated with ORKAMBI and ivacaftor, a component of ORKAMBI. Baseline and follow-up examinations are recommended in pediatric patients initiating ORKAMBI. (5.7)
Adverse Reactions/Side Effects
The most common adverse reactions to ORKAMBI (occurring in ≥5% of patients with CF homozygous for the F508del mutation in the CFTR gene) were dyspnea, nasopharyngitis, nausea, diarrhea, upper respiratory tract infection, fatigue, respiration abnormal, blood creatine phosphokinase increased, rash, flatulence, rhinorrhea, influenza. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Vertex Pharmaceuticals Incorporated at 1-877-634-8789 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
See Full Prescribing Information for a complete list. (2.3, 7, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2023
Related/similar drugs
azithromycin, Zithromax, gentamicin, Creon, tobramycinFull Prescribing Information
1. Indications and Usage for Orkambi
ORKAMBI is indicated for the treatment of cystic fibrosis (CF) in patients aged 1 year and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of the F508del mutation on both alleles of the CFTR gene.
2. Orkambi Dosage and Administration
2.1 Recommended Dosage in Adults and Pediatric Patients Aged 1 Year and Older
The recommended dosage of ORKAMBI in adults and pediatric patients aged one year and older is based on patient's age and weight as described in Table 1.
| Age Group | Weight | ORKAMBI Daily Dose (every 12 hours) | |
|---|---|---|---|
| Morning Dose | Evening Dose | ||
| 1 through 2 years | 7 kg to <9 kg | 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules | 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules |
| 9 kg to <14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules | |
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | |
| 2 through 5 years | <14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules |
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | |
| 6 through 11 years | - | 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg (lumacaftor 200 mg/ivacaftor 250 mg per dose) | 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg (lumacaftor 200 mg/ivacaftor 250 mg per dose) |
| 12 years and older | - | 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg (lumacaftor 400 mg/ivacaftor 250 mg per dose) | 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg (lumacaftor 400 mg/ivacaftor 250 mg per dose) |
2.2 Dosage Adjustment for Patients with Hepatic Impairment
For dose adjustment for patients with hepatic impairment, refer to Table 2.
Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C), but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a maximum dose of 1 tablet in the morning and 1 tablet in the evening or less frequently, or 1 packet of oral granules once daily or less frequently in patients with severe hepatic impairment after weighing the risks and benefits of treatment [see Dosage and Administration (2.1), Use in Specific Populations (8.6), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
| Age Group | Weight | Morning Dose | Evening Dose | |
|---|---|---|---|---|
|
||||
| Mild (Child-Pugh Class A) | 1 through 2 years | 7 kg to <9 kg | 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules | 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules |
| 9 kg to <14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules | ||
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | ||
| 2 through 5 years | <14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules | |
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | ||
| 6 through 11 years | - | 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg (lumacaftor 200 mg/ivacaftor 250 mg per dose) | 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg (lumacaftor 200 mg/ivacaftor 250 mg per dose) |
|
| 12 years and older | - | 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg (lumacaftor 400 mg/ivacaftor 250 mg per dose) | 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg (lumacaftor 400 mg/ivacaftor 250 mg per dose) |
|
| Moderate (Child-Pugh Class B) | 1 through 2 years | 7 kg to <9 kg | 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules | 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules every other day |
| 9 kg to <14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules every other day | ||
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules every other day | ||
| 2 through 5 years | <14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules every other day | |
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules every other day | ||
| 6 through 11 years | - | 2 tablets of lumacaftor 100 mg/ivacaftor 125 mg (lumacaftor 200 mg/ivacaftor 250 mg per dose) | 1 tablet of lumacaftor 100 mg/ivacaftor 125 mg | |
| 12 years and older | - | 2 tablets of lumacaftor 200 mg/ivacaftor 125 mg (lumacaftor 400 mg/ivacaftor 250 mg per dose) | 1 tablet of lumacaftor 200 mg/ivacaftor 125 mg | |
| Severe (Child-Pugh Class C) | 1 through 2 years | 7 kg to <9 kg | 1 packet of lumacaftor 75 mg/ivacaftor 94 mg oral granules* | N/A |
| 9 kg to <14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules* | |||
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules* | |||
| 2 through 5 years | <14 kg | 1 packet of lumacaftor 100 mg/ivacaftor 125 mg oral granules* | ||
| ≥14 kg | 1 packet of lumacaftor 150 mg/ivacaftor 188 mg oral granules* | |||
| 6 through 11 years | - | 1 tablet of lumacaftor 100 mg/ivacaftor 125 mg* | 1 tablet of lumacaftor 100 mg/ivacaftor 125 mg* | |
| 12 years and older | - | 1 tablet of lumacaftor 200 mg/ivacaftor 125 mg* | 1 tablet of lumacaftor 200 mg/ivacaftor 125 mg* | |
2.3 Dosage Adjustment for Patients Taking CYP3A Inhibitors
No dose adjustment is necessary when CYP3A inhibitors are initiated in patients already taking ORKAMBI. However, when initiating ORKAMBI in patients currently taking strong CYP3A inhibitors, reduce the ORKAMBI dosage for the first week of treatment based on age as follows [see Dosage and Administration (2.1) and Drug Interactions (7.1)]:
- 1 through 5 years of age: 1 packet of granules every other day
- 6 years of age and older: 1 tablet daily
Following this one-week period, resume the recommended daily dosage.
If ORKAMBI is interrupted for more than one-week and then re-initiated while taking strong CYP3A inhibitors, reduce the ORKAMBI dosage for the first week of treatment re-initiation based on age as follows:
- 1 through 5 years of age: 1 packet of granules every other day
- 6 years of age and older: 1 tablet daily
Following this one-week period, resume the recommended daily dosage.
3. Dosage Forms and Strengths
- Tablets: 100 mg lumacaftor and 125 mg ivacaftor; supplied as pink, oval-shaped, film-coated, fixed-dose combination tablets containing 100 mg of lumacaftor and 125 mg of ivacaftor. Each tablet is printed with the characters "1V125" in black ink on one side and plain on the other.
- Tablets: 200 mg lumacaftor and 125 mg ivacaftor; supplied as pink, oval-shaped, film-coated, fixed-dose combination tablets containing 200 mg of lumacaftor and 125 mg of ivacaftor. Each tablet is printed with the characters "2V125" in black ink on one side and plain on the other.
- Oral granules: Unit-dose packets containing lumacaftor 75 mg/ivacaftor 94 mg or lumacaftor 100 mg/ivacaftor 125 mg or lumacaftor 150 mg/ivacaftor 188 mg per packet; supplied as small, white to off-white granules in unit-dose packets.
5. Warnings and Precautions
5.1 Use in Patients with Advanced Liver Disease
Worsening of liver function, including hepatic encephalopathy, in patients with advanced liver disease has been reported. Liver function decompensation, including liver failure leading to death, has been reported in CF patients with pre-existing cirrhosis with portal hypertension while receiving ORKAMBI. Use ORKAMBI with caution in patients with advanced liver disease and only if the benefits are expected to outweigh the risks. If ORKAMBI is used in these patients, they should be closely monitored after the initiation of treatment and the dose should be reduced [see Dosage and Administration (2.2) and Adverse Reactions (6.1)].
5.2 Liver-related Events
Serious adverse reactions related to elevated transaminases have been reported in patients with CF receiving ORKAMBI. In some instances, these elevations have been associated with concomitant elevations in total serum bilirubin.
It is recommended that ALT, AST, and bilirubin be assessed prior to initiating ORKAMBI, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of ALT, AST, or bilirubin elevations, more frequent monitoring should be considered. Patients who develop increased ALT, AST, or bilirubin should be closely monitored until the abnormalities resolve.
Dosing should be interrupted in patients with ALT or AST >5 × upper limit of normal (ULN) when not associated with elevated bilirubin. Dosing should also be interrupted in patients with ALT or AST elevations >3 × ULN when associated with bilirubin elevations >2 × ULN. Following resolution of transaminase elevations, consider the benefits and risks of resuming dosing [see Adverse Reactions (6.1)].
5.3 Hypersensitivity Reactions, Including Anaphylaxis
Hypersensitivity reactions, including cases of angioedema and anaphylaxis, have been reported in the postmarketing setting [see Adverse Reactions (6.2)]. If signs or symptoms of serious hypersensitivity reactions develop during treatment, discontinue ORKAMBI and institute appropriate therapy. Consider the benefits and risks for the individual patient to determine whether to resume treatment with ORKAMBI.
5.4 Respiratory Events
Respiratory events (e.g., chest discomfort, dyspnea, and respiration abnormal) were observed more commonly in patients during initiation of ORKAMBI compared to those who received placebo. These events have led to drug discontinuation and can be serious, particularly in patients with advanced lung disease (percent predicted FEV1 <40). Clinical experience in patients with ppFEV1 <40 is limited, and additional monitoring of these patients is recommended during initiation of therapy [see Adverse Reactions (6.1)].
5.5 Effect on Blood Pressure
Increased blood pressure has been observed in some patients treated with ORKAMBI. Blood pressure should be monitored periodically in all patients being treated with ORKAMBI [see Adverse Reactions (6.1)].
5.7 Cataracts
Cases of non-congenital lens opacities have been reported in pediatric patients treated with ORKAMBI and ivacaftor, a component of ORKAMBI. Although other risk factors were present in some cases (such as corticosteroid use and exposure to radiation), a possible risk attributable to ivacaftor cannot be excluded [see Use in Specific Populations (8.4)]. Baseline and follow-up ophthalmological examinations are recommended in pediatric patients initiating ORKAMBI treatment.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the label:
- Use in Patients with Advanced Liver Disease [see Warnings and Precautions (5.1)]
- Liver-related Events [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions, Including Anaphylaxis [see Warnings and Precautions (5.3)]
- Respiratory Events [see Warnings and Precautions (5.4)]
- Effect on Blood Pressure [see Warnings and Precautions (5.5)]
- Cataracts [see Warnings and Precautions (5.7)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The overall safety profile of ORKAMBI is based on the pooled data from 1108 patients with CF aged 12 years and older who are homozygous for the F508del mutation in the CFTR gene and who received at least one dose of study drug in two double-blind, placebo-controlled, Phase 3 clinical trials, each with 24 weeks of treatment (Trials 1 and 2).
In addition, the following clinical trials have been conducted:
- A 24-week, open-label trial (Trial 3) in 58 patients with CF aged 6 through 11 years homozygous for the F508del-CFTR mutation.
- A 24-week, placebo-controlled trial (Trial 4) in 204 patients aged 6 through 11 years homozygous for the F508del-CFTR mutation.
- A 24-week, open-label trial (Trial 5) in 46 patients aged 12 years and older homozygous for the F508del-CFTR mutation and with advanced lung disease (ppFEV1 <40).
- A 24-week, open-label trial (Trial 6) in 60 patients aged 2 through 5 years homozygous for the F508del-CFTR mutation.
- A 24-week, open-label trial (Trial 7) in 46 patients aged 1 through 2 years homozygous for the F508del-CFTR mutation.
Of the 1108 patients, in the pooled analyses of Trial 1 and Trial 2, 49% were female and 99% were Caucasian; 369 patients received ORKAMBI every 12 hours and 370 patients received placebo.
The proportion of patients who prematurely discontinued study drug due to adverse events was 5% for patients treated with ORKAMBI and 2% for patients who received placebo.
Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in patients treated with ORKAMBI included pneumonia, hemoptysis, cough, increased blood creatine phosphokinase, and transaminase elevations. These occurred in 1% or less of patients.
Table 3 shows adverse reactions occurring in ≥5% of patients with CF aged 12 years and older treated with ORKAMBI who are homozygous for the F508del mutation in the CFTR gene that also occurred at a higher rate than in patients who received placebo in the two double-blind, placebo-controlled trials.
| Adverse Reaction (Preferred Term) | ORKAMBI N=369 (%) | Placebo N=370 (%) |
|---|---|---|
| Dyspnea | 48 (13) | 29 (8) |
| Nasopharyngitis | 48 (13) | 40 (11) |
| Nausea | 46 (13) | 28 (8) |
| Diarrhea | 45 (12) | 31 (8) |
| Upper respiratory tract infection | 37 (10) | 20 (5) |
| Fatigue | 34 (9) | 29 (8) |
| Respiration abnormal | 32 (9) | 22 (6) |
| Blood creatine phosphokinase increased | 27 (7) | 20 (5) |
| Rash | 25 (7) | 7 (2) |
| Flatulence | 24 (7) | 11 (3) |
| Rhinorrhea | 21 (6) | 15 (4) |
| Influenza | 19 (5) | 8 (2) |
The safety profile from two pediatric trials in CF patients aged 6 through 11 years who are homozygous for the F508del-CFTR mutation, a 24-week, open-label, multicenter safety trial in 58 patients (Trial 3) and a 24-week, placebo-controlled, clinical trial (Trial 4) in 204 patients (103 received lumacaftor 200 mg/ivacaftor 250 mg every 12 hours and 101 received placebo), was similar to that observed in Trials 1 and 2. Adverse reactions that are not listed in Table 3, and that occurred in ≥5% of lumacaftor/ivacaftor-treated patients with an incidence of ≥3% higher than placebo included: productive cough (17.5% vs 5.9%), nasal congestion (16.5% vs 7.9%), headache (12.6% vs 8.9%), abdominal pain upper (12.6% vs 6.9%), and sputum increased (10.7% vs 2.0%).
In a 24-week, open-label, multicenter, study in 60 patients aged 2 through 5 years with CF who are homozygous for the F508del-CFTR mutation (Trial 6) the safety profile was similar to that observed in studies in patients aged 6 years and older [see Clinical Pharmacology (12.2)].
In a 24-week, open-label, multicenter, study in 46 patients aged 1 through 2 years with CF who are homozygous for the F508del-CFTR mutation (Trial 7) the safety profile was similar to that observed in studies in patients aged 2 years and older [see Clinical Pharmacology (12.2)].
Additional information on selected adverse reactions from trials is detailed below:
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of ORKAMBI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hepatobiliary: liver function decompensation including liver failure leading to death in patients with pre-existing cirrhosis with portal hypertension [see Warnings and Precautions (5.1)].
Immune System Disorders: anaphylaxis, angioedema
7. Drug Interactions
7.1 Inhibitors of CYP3A
Co-administration of lumacaftor/ivacaftor with itraconazole, a strong CYP3A inhibitor, did not impact the exposure of lumacaftor, but increased ivacaftor exposure by 4.3-fold. Due to the induction effect of lumacaftor on CYP3A, at steady-state, the net exposure of ivacaftor is not expected to exceed that when given in the absence of lumacaftor at a dose of 150 mg every 12 hours (the approved dose of ivacaftor monotherapy). Therefore, no dose adjustment is necessary when CYP3A inhibitors are initiated in patients currently taking ORKAMBI. However, when initiating ORKAMBI in patients taking strong CYP3A inhibitors, reduce the ORKAMBI dosage as recommended for the first week of treatment to allow for the steady-state induction effect of lumacaftor. Following this period, continue with the recommended daily dose [see Dosage and Administration (2.3)].
Examples of strong CYP3A inhibitors include:
- ketoconazole, itraconazole, posaconazole, and voriconazole.
- telithromycin, clarithromycin.
No dose adjustment is recommended when used with moderate or weak CYP3A inhibitors.
7.2 Inducers of CYP3A
Co-administration of lumacaftor/ivacaftor with rifampin, a strong CYP3A inducer, had minimal effect on the exposure of lumacaftor, but decreased ivacaftor exposure (AUC) by 57%. This may reduce the effectiveness of ORKAMBI. Therefore, co-administration with strong CYP3A inducers, such as rifampin, rifabutin, phenobarbital, carbamazepine, phenytoin, and St. John's wort (Hypericum perforatum), is not recommended [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)].
No dose adjustment is recommended when used with moderate or weak CYP3A inducers.
7.3 CYP3A Substrates
Lumacaftor is a strong inducer of CYP3A. Co-administration of lumacaftor with ivacaftor, a sensitive CYP3A substrate, decreased ivacaftor exposure by approximately 80%. Administration of ORKAMBI may decrease systemic exposure of medicinal products which are substrates of CYP3A, thereby decreasing the therapeutic effect of the medicinal product.
Co-administration of ORKAMBI is not recommended with sensitive CYP3A substrates or CYP3A substrates with a narrow therapeutic index [see Warnings and Precautions (5.6) and Clinical Pharmacology (12.3)] such as:
- Benzodiazepines: midazolam, triazolam (consider an alternative to these benzodiazepines).
- Immunosuppressants: cyclosporine, everolimus, sirolimus, and tacrolimus (avoid the use of ORKAMBI).
7.4 CYP2B6 and CYP2C Substrates
In vitro studies suggest that lumacaftor has the potential to induce CYP2B6, CYP2C8, CYP2C9, and CYP2C19; inhibition of CYP2C8 and CYP2C9 has also been observed in vitro. Additionally, in vitro studies suggest that ivacaftor may inhibit CYP2C9. Therefore, concomitant use of ORKAMBI with CYP2B6, CYP2C8, CYP2C9, and CYP2C19 substrates may alter the exposure of these substrates.
7.5 Digoxin and Other P-gp Substrates
Based on in vitro results which showed P-gp inhibition and pregnane-X-receptor (PXR) activation, lumacaftor has the potential to both inhibit and induce P-gp. Additionally, a clinical study with ivacaftor monotherapy showed that ivacaftor is a weak inhibitor of P-gp. Therefore, concomitant use of ORKAMBI with P-gp substrates may alter the exposure of these substrates.
Monitor the serum concentration of digoxin and titrate the digoxin dose to obtain the desired clinical effect.
7.6 Anti-allergics and Systemic Corticosteroids
ORKAMBI may decrease the exposure of montelukast, which may reduce its efficacy. No dose adjustment for montelukast is recommended. Employ appropriate clinical monitoring, as is reasonable, when co-administered with ORKAMBI.
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of prednisone and methylprednisolone. A higher dose of these systemic corticosteroids may be required to obtain the desired clinical effect.
7.7 Antibiotics
Concomitant use of ORKAMBI may decrease the exposure of clarithromycin, erythromycin, and telithromycin, which may reduce the effectiveness of these antibiotics. Consider an alternative to these antibiotics, such as ciprofloxacin, azithromycin, and levofloxacin.
7.8 Antifungals
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of itraconazole, ketoconazole, posaconazole, and voriconazole. Concomitant use of ORKAMBI with these antifungals is not recommended. Monitor patients closely for breakthrough fungal infections if such drugs are necessary. Consider an alternative such as fluconazole.
7.9 Anti-inflammatories
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of ibuprofen. A higher dose of ibuprofen may be required to obtain the desired clinical effect.
7.10 Antidepressants
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of citalopram, escitalopram, and sertraline. A higher dose of these antidepressants may be required to obtain the desired clinical effect.
7.11 Hormonal Contraceptives
ORKAMBI may decrease hormonal contraceptive exposure, reducing the effectiveness. Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with ORKAMBI.
Concomitant use of ORKAMBI with hormonal contraceptives increased the menstrual abnormality events [see Adverse Reactions (6.1)]. Avoid concomitant use unless the benefit outweighs the risks.
7.12 Oral Hypoglycemics
Concomitant use of ORKAMBI may reduce the exposure and effectiveness of repaglinide and may alter the exposure of sulfonylurea. A dose adjustment may be required to obtain the desired clinical effect. No dose adjustment is recommended for metformin.
7.14 Warfarin
ORKAMBI may alter the exposure of warfarin. Monitor the international normalized ratio (INR) when warfarin co-administration with ORKAMBI is required.
7.15 Concomitant Drugs That Do Not Need Dose Adjustment
No dosage adjustment of ORKAMBI or concomitant drug is recommended when ORKAMBI is given with the following: azithromycin, aztreonam, budesonide, ceftazidime, cetirizine, ciprofloxacin, colistimethate, colistin, dornase alfa, fluticasone, ipratropium, levofloxacin, pancreatin, pancrelipase, salbutamol, salmeterol, sulfamethoxazole, trimethoprim, tiotropium, and tobramycin. Based on the metabolism and route of elimination, ORKAMBI is not expected to impact the exposure of these drugs.
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
ORKAMBI may decrease hormonal contraceptive exposure, reducing the effectiveness. Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with ORKAMBI [see Warnings and Precautions (5.6) and Drug Interactions (7.11)].
8.4 Pediatric Use
The safety and effectiveness of ORKAMBI in pediatric patients one year of age and older have been established. Use of ORKAMBI in these age groups is supported by evidence from adequate and well-controlled studies of ORKAMBI in patients 12 years of age and older [see Clinical Studies (14) and Adverse Reactions (6.1)] with additional data as follows:
- Extrapolation of efficacy in patients aged 12 years and older homozygous for the F508del mutation in the CFTR gene to pediatric patients aged 1 through 11 years with support from population pharmacokinetic analyses showing similar drug exposure levels in patients aged 12 years and older and in patients aged 1 through 11 years [see Clinical Pharmacology (12.3)].
- Safety data were obtained from a 24-week, open-label, clinical trial in 58 patients aged 6 through 11 years, mean age 9 years (Trial 3) and a 24-week, placebo-controlled, clinical trial in 204 patients aged 6 through 11 years (Trial 4). Trial 3 evaluated subjects with a screening ppFEV1 ≥40 [mean ppFEV1 91.4 at baseline (range: 55 to 122.7)]. Trial 4 evaluated subjects with a screening ppFEV1 ≥70 [mean ppFEV1 89.8 at baseline (range: 48.6 to 119.6)]. The safety profile of ORKAMBI in pediatric patients 6 through 11 years of age was similar to that in patients aged 12 years and older [see Adverse Reactions (6.1)]. In Trial 3, spirometry (ppFEV1) was assessed as a planned safety endpoint. The within-group LS mean absolute change from baseline in ppFEV1 at Week 24 was 2.5 percentage points. At the Week 26 safety follow-up visit (following a planned discontinuation) ppFEV1 was also assessed. The within-group LS mean absolute change in ppFEV1 from Week 24 at Week 26 was -3.2 percentage points.
- Additional safety data were obtained from Trial 6, a 24-week, open-label, clinical trial in 60 patients aged 2 through 5 years at screening (mean age at baseline 3.7 years). The safety profile in Trial 6 was similar to that in patients aged 6 years and older [see Adverse Reactions (6.1)].
- Additional safety data were obtained from Trial 7, a 24-week, open-label, clinical trial in 46 patients aged 1 to 2 years at screening (mean age at baseline 18.1 months). The safety profile in Trial 7 was similar to that in patients aged 2 years and older [see Adverse Reactions (6.1)].
The safety and effectiveness of ORKAMBI in patients with CF younger than 1 year of age have not been established.
Cases of non-congenital lens opacities have been reported in pediatric patients treated with ORKAMBI and ivacaftor, a component of ORKAMBI. Although other risk factors were present in some cases (such as corticosteroid use and exposure to radiation), a possible risk attributable to ivacaftor cannot be excluded [see Warnings and Precautions (5.7)].
8.5 Geriatric Use
CF is largely a disease of children and young adults. Clinical trials of ORKAMBI did not include sufficient numbers of patients 65 years of age and over to determine whether they respond differently from younger patients.
8.6 Hepatic Impairment
No dose adjustment is necessary for patients with mild hepatic impairment (Child-Pugh Class A). A dose reduction to 2 tablets in the morning and 1 tablet in the evening is recommended for patients aged 6 years and older with moderate hepatic impairment (Child-Pugh Class B). A dose reduction to 1 packet of oral granules in the morning daily and 1 packet of oral granules in the evening every other day is recommended for patients aged 1 to 5 years old with moderate hepatic impairment (Child-Pugh Class B).
Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C), but exposure is expected to be higher than in patients with moderate hepatic impairment. Therefore, use with caution at a maximum dose of 1 tablet in the morning and 1 tablet in the evening or less frequently, or 1 packet of oral granules once daily or less frequently in patients with severe hepatic impairment after weighing the risks and benefits of treatment [see Warnings and Precautions (5.1), Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Patient Counseling Information (17)].
8.7 Renal Impairment
ORKAMBI has not been studied in patients with mild, moderate, or severe renal impairment or in patients with end-stage renal disease. No dose adjustment is necessary for patients with mild to moderate renal impairment. Caution is recommended while using ORKAMBI in patients with severe renal impairment (creatinine clearance ≤30 mL/min) or end-stage renal disease.
10. Overdosage
There have been no reports of overdose with ORKAMBI.
The highest repeated dose was lumacaftor 1000 mg once daily/ivacaftor 450 mg q12h administered to 49 healthy subjects for 7 days in a trial evaluating the effect of ORKAMBI on electrocardiograms (ECGs). Adverse events reported at an increased incidence of ≥5% compared to the lumacaftor 600 mg/ivacaftor 250 mg dosing period and placebo included: headache (29%), transaminase increased (18%), and generalized rash (10%).
No specific antidote is available for overdose with ORKAMBI. Treatment of overdose consists of general supportive measures including monitoring of vital signs and observation of the clinical status of the patient.
11. Orkambi Description
The active ingredients in ORKAMBI tablets are lumacaftor, which has the following chemical name: 3-[6-({[1-(2,2-difluoro-1,3-benzodioxol-5-yl)cyclopropyl]carbonyl}amino)-3-methylpyridin-2-yl]benzoic acid, and ivacaftor, a CFTR potentiator, which has the following chemical name: N-(2,4-di-tert-butyl-5-hydroxyphenyl)-1,4-dihydro-4-oxoquinoline-3-carboxamide. The molecular formula for lumacaftor is C24H18F2N2O5 and for ivacaftor is C24H28N2O3. The molecular weights for lumacaftor and ivacaftor are 452.41 and 392.49, respectively. The structural formulas are:
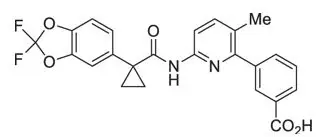
lumacaftor

ivacaftor
Lumacaftor is a white to off-white powder that is practically insoluble in water (0.02 mg/mL). Ivacaftor is a white to off-white powder that is practically insoluble in water (<0.05 µg/mL).
ORKAMBI is available as a pink, oval-shaped, film-coated tablet for oral administration containing 200 mg of lumacaftor and 125 mg of ivacaftor. Each ORKAMBI tablet contains 200 mg of lumacaftor and 125 mg of ivacaftor, and the following inactive ingredients: cellulose, microcrystalline; croscarmellose sodium; hypromellose acetate succinate; magnesium stearate; povidone; and sodium lauryl sulfate. The tablet film coat contains carmine, FD&C Blue #1, FD&C Blue #2, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. The printing ink contains ammonium hydroxide, iron oxide black, propylene glycol, and shellac.
ORKAMBI is also available as a pink, oval-shaped, film-coated tablet for oral administration containing 100 mg of lumacaftor and 125 mg of ivacaftor. Each ORKAMBI tablet contains 100 mg of lumacaftor and 125 mg of ivacaftor, and the following inactive ingredients: cellulose, microcrystalline; croscarmellose sodium; hypromellose acetate succinate; magnesium stearate; povidone; and sodium lauryl sulfate. The tablet film coat contains carmine, FD&C Blue #1, FD&C Blue #2, polyethylene glycol, polyvinyl alcohol, talc, and titanium dioxide. The printing ink contains ammonium hydroxide, iron oxide black, propylene glycol, and shellac.
ORKAMBI is also available as white to off-white granules for oral administration and enclosed in a unit-dose packet containing lumacaftor 75 mg/ivacaftor 94 mg, lumacaftor 100 mg/ivacaftor 125 mg or lumacaftor 150 mg/ivacaftor 188 mg per packet. Each unit-dose packet of ORKAMBI oral granules contains lumacaftor 75 mg/ivacaftor 94 mg, lumacaftor 100 mg/ivacaftor 125 mg or lumacaftor 150 mg/ivacaftor 188 mg per packet and the following inactive ingredients: cellulose, microcrystalline; croscarmellose sodium; hypromellose acetate succinate; povidone; and sodium lauryl sulfate.
12. Orkambi - Clinical Pharmacology
12.1 Mechanism of Action
The CFTR protein is a chloride channel present at the surface of epithelial cells in multiple organs. The F508del mutation results in protein misfolding, causing a defect in cellular processing and trafficking that targets the protein for degradation and therefore reduces the quantity of CFTR at the cell surface. The small amount of F508del-CFTR that reaches the cell surface is less stable and has low channel-open probability (defective gating activity) compared to wild-type CFTR protein.
Lumacaftor improves the conformational stability of F508del-CFTR, resulting in increased processing and trafficking of mature protein to the cell surface. Ivacaftor is a CFTR potentiator that facilitates increased chloride transport by potentiating the channel-open probability (or gating) of the CFTR protein at the cell surface. In vitro studies have demonstrated that both lumacaftor and ivacaftor act directly on the CFTR protein in primary human bronchial epithelial cultures and other cell lines harboring the F508del-CFTR mutation to increase the quantity, stability, and function of F508del-CFTR at the cell surface, resulting in increased chloride ion transport. In vitro responses do not necessarily correspond to in vivo pharmacodynamic response or clinical benefit.
12.3 Pharmacokinetics
The exposure (AUC) of lumacaftor is approximately 2-fold higher in healthy adult volunteers compared to exposure in patients with CF. The exposure of ivacaftor is similar between healthy adult volunteers and patients with CF. After twice daily dosing, steady-state plasma concentrations of lumacaftor and ivacaftor in healthy subjects were generally reached after approximately 7 days of treatment, with an accumulation ratio of approximately 1.9 for lumacaftor. The steady-state exposure of ivacaftor is lower than that of Day 1 due to the CYP3A induction effect of lumacaftor.
| Drug | Cmax
(μg/mL) | t½*
(h) | AUC0-12h
(μg∙h/mL) |
|
|---|---|---|---|---|
|
||||
| Lumacaftor 400 mg q12h/
Ivacaftor 250 mg q12h | Lumacaftor | 25.0 (7.96) | 25.2 (9.94) | 198 (64.8) |
| Ivacaftor | 0.602 (0.304) | 9.34 (3.81) | 3.66 (2.25) | |
Specific Populations
Pediatric Patients
The following conclusions about exposures between adults and the pediatric population are based on population pharmacokinetics (PK) analyses:
| Age Group | Weight | Dose | Mean Lumacaftor (SD)*
AUCss (µg∙h/mL) | Mean Ivacaftor (SD)†
AUCss (µg∙h/mL) |
|---|---|---|---|---|
|
||||
| Patients aged 1 to <2 years | 7 kg to <9 kg | lumacaftor 75 mg/ivacaftor 94 mg every 12 hours. | 234 | 7.98 |
| 9 kg to <14 kg | lumacaftor 100 mg/ivacaftor 125 mg every 12 hours. | 191 (40.6) | 5.35 (1.61) | |
| ≥14 kg | lumacaftor 150 mg/ivacaftor 188 mg every 12 hours. | 116 | 5.82 | |
| Patients aged 2 through 5 years | <14 kg | lumacaftor 100 mg/ivacaftor 125 mg every 12 hours. | 180 (45.5) | 5.92 (4.61) |
| ≥14 kg | lumacaftor 150 mg/ivacaftor 188 mg every 12 hours. | 217 (48.6) | 5.90 (1.93) | |
| Patients aged 6 through 11 years | - | lumacaftor 200 mg/ivacaftor 250 mg every 12 hours. | 203 (57.4) | 5.26 (3.08) |
| Patients aged 12 to <18 years | - | lumacaftor 400 mg/ivacaftor 250 mg every 12 hours. | 241 (61.4) | 3.90 (1.56) |
Drug Interaction Studies
Drug interaction studies were performed with lumacaftor/ivacaftor and other drugs likely to be co-administered or drugs commonly used as probes for pharmacokinetic interaction studies [see Drug Interactions (7)].
Potential for Other Drugs to Affect Lumacaftor/Ivacaftor
Lumacaftor exposure is not affected by concomitant CYP3A inducers or inhibitors. Exposure of ivacaftor when given in combination with lumacaftor is reduced by concomitant CYP3A inducers and increased by concomitant CYP3A inhibitors [see Dosage and Administration (2.3), Warnings and Precautions (5.6), and Drug Interactions (7)].
The effects of co-administered drugs on the exposure of lumacaftor and ivacaftor are shown in Table 6 [see Dosage and Administration (2.3), Warnings and Precautions (5.6), and Drug Interactions (7)].
| Co-administered Drug | Dose of Co-administered Drug | Effect on PK* | Mean Ratio (90% CI) of Lumacaftor and Ivacaftor No Effect=1.0 |
|
|---|---|---|---|---|
| AUC | Cmax | |||
| CI = Confidence Interval; PK = Pharmacokinetics. | ||||
|
||||
| CYP3A inhibitor: itraconazole | 200 mg once daily | ↔ Lumacaftor | 0.97 (0.91, 1.02) | 0.99 (0.92, 1.05) |
| ↑ Ivacaftor | 4.30†
(3.78, 4.88) | 3.64†
(3.19, 4.17) |
||
| CYP3A inducer: rifampin | 600 mg once daily | ↔ Lumacaftor | 0.87 (0.81, 0.93) | 0.96 (0.87, 1.05) |
| ↓ Ivacaftor | 0.43 (0.38, 0.49) | 0.50 (0.43, 0.58) |
||
| Other: ciprofloxacin | 750 mg q12h | ↔ Lumacaftor | 0.86 (0.79, 0.95) | 0.88 (0.80, 0.97) |
| ↔ Ivacaftor | 1.29 (1.12, 1.48) | 1.29 (1.11, 1.49) |
||
13. Nonclinical Toxicology
14. Clinical Studies
Confirmatory
The efficacy of ORKAMBI in patients with CF who are homozygous for the F508del mutation in the CFTR gene was evaluated in two randomized, double-blind, placebo-controlled, 24-week clinical trials (Trials 1 and 2) in 1108 clinically stable patients with CF of whom 369 patients received ORKAMBI twice daily.
Trial 1 evaluated 549 patients with CF who were aged 12 years and older (mean age 25.1 years) with ppFEV1 at screening between 40-90 [mean ppFEV1 60.7 at baseline (range: 31.1 to 94.0)]. Trial 2 evaluated 559 patients aged 12 years and older (mean age 25.0 years) with ppFEV1 at screening between 40-90 [mean ppFEV1 60.5 at baseline (range: 31.3 to 99.8)]. Patients with a history of colonization with organisms such as Burkholderia cenocepacia, Burkholderia dolosa, or Mycobacterium abscessus, or who had 3 or more abnormal liver function tests (ALT, AST, AP, GGT ≥3 × the ULN or total bilirubin ≥2 × the ULN) were excluded.
Patients in both trials were randomized 1:1:1 to receive either ORKAMBI (lumacaftor 400 mg q12h/ivacaftor 250 mg q12h; or lumacaftor 600 mg once daily/ivacaftor 250 mg q12h) or placebo. Patients took the study drug with fat-containing food for 24 weeks in addition to their prescribed CF therapies (e.g., bronchodilators, inhaled antibiotics, dornase alfa, and hypertonic saline).
The primary efficacy endpoint in both trials was change in lung function as determined by absolute change from baseline in ppFEV1 at Week 24, assessed as the average of the treatment effects at Week 16 and at Week 24. In both trials, treatment with ORKAMBI resulted in a statistically significant improvement in ppFEV1. The treatment difference between ORKAMBI and placebo for the mean absolute change in ppFEV1 from baseline at Week 24 (assessed as the average of the treatment effects at Week 16 and at Week 24) was 2.6 percentage points [95% CI (1.2, 4.0)] in Trial 1 (P=0.0003) and 3.0 percentage points [95% CI (1.6, 4.4)] in Trial 2 (P<0.0001). These changes persisted throughout the 24-week treatment period (see Figure 1). Improvements in ppFEV1 were observed regardless of age, disease severity, sex, and geographic region.
| Figure 1. Absolute Change From Baseline at Each Visit in Percent Predicted FEV1 in Trial 1 and Trial 2. | |
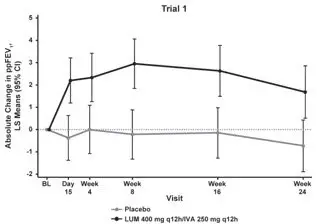 | 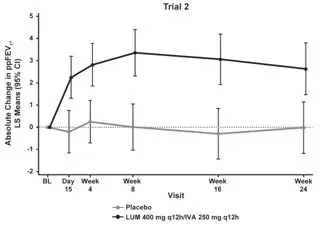 |
| LS = Least Squares; q12h = every 12 hours | |
Key secondary efficacy variables included relative change from baseline in ppFEV1 at Week 24, assessed as the average of the treatment effects at Week 16 and at Week 24; absolute change from baseline in BMI at Week 24; absolute change from baseline in Cystic Fibrosis Questionnaire-Revised (CFQ-R) Respiratory Domain score at Week 24, a measure of respiratory symptoms relevant to patients with CF such as cough, sputum production, and difficulty breathing; proportion of patients achieving ≥5% relative change from baseline in ppFEV1 using the average of Week 16 and Week 24; and number of pulmonary exacerbations through Week 24. For the purposes of these trials, a pulmonary exacerbation was defined as a change in antibiotic therapy (IV, inhaled, or oral) as a result of 4 or more of 12 pre-specified sino-pulmonary signs/symptoms.
| Trial 1 | Trial 2 | ||||
|---|---|---|---|---|---|
| Placebo (n=184) | ORKAMBI LUM 400 mg q12h/IVA 250 mg q12h (n=182) | Placebo (n=187) | ORKAMBI LUM 400 mg q12h/IVA 250 mg q12h (n=187) |
||
|
|||||
| Relative change in ppFEV1 at Week 24† (%) | Treatment difference (95% CI) | – | 4.3 (1.9, 6.8) P=0.0006‡ | – | 5.3 (2.7, 7.8) P<0.0001‡ |
| Absolute change in BMI at Week 24 (kg/m2) | Treatment difference (95% CI) | – | 0.1 (-0.1, 0.3) | – | 0.4 (0.2, 0.5) P=0.0001‡ |
| Absolute change in CFQ-R Respiratory Domain Score (Points) at Week 24 | Treatment difference (95% CI) | – | 1.5 (-1.7, 4.7) | – | 2.9 (-0.3, 6.0) |
| Proportion of patients with ≥5% relative change in ppFEV1 at Week 24† | % | 22% | 37% | 23% | 41% |
| Odds ratio (95% CI) | – | 2.1 (1.3, 3.3) | – | 2.4 (1.5, 3.7) |
|
| Number of pulmonary exacerbations through Week 24 | # of events (rate per 48 weeks) | 112 (1.1) | 73 (0.7) | 139 (1.2) | 79 (0.7) |
| Rate ratio (95% CI) | – | 0.7 (0.5, 0.9) | – | 0.6 (0.4, 0.8) |
|
16. How is Orkambi supplied
ORKAMBI (lumacaftor 200 mg/ivacaftor 125 mg) is supplied as pink, oval-shaped tablets; each tablet contains 200 mg of lumacaftor and 125 mg of ivacaftor, printed with "2V125" in black ink on one side and plain on the other, and is packaged as follows:
| 112–count tablet box containing a 4-week supply (4 weekly cartons of 7 daily blister strips with 4 tablets per strip). | NDC 51167-809-01 |
ORKAMBI (lumacaftor 100 mg/ivacaftor 125 mg) is supplied as pink, oval-shaped tablets; each tablet contains 100 mg of lumacaftor and 125 mg of ivacaftor, printed with "1V125" in black ink on one side and plain on the other, and is packaged as follows:
| 112–count tablet box containing a 4-week supply (4 weekly cartons of 7 daily blister strips with 4 tablets per strip). | NDC 51167-700-02 |
ORKAMBI (lumacaftor/ivacaftor) oral granules are supplied as small white to off-white granules and enclosed in unit-dose packets as follows:
| 56-count carton (contains 56 unit-dose packets of lumacaftor 75 mg/ivacaftor 94 mg per packet) | NDC 51167-122-01 |
| 56-count carton (contains 56 unit-dose packets of lumacaftor 100 mg/ivacaftor 125 mg per packet) | NDC 51167-900-01 |
| 56-count carton (contains 56 unit-dose packets of lumacaftor 150 mg/ivacaftor 188 mg per packet) | NDC 51167-500-02 |
| ORKAMBI
lumacaftor and ivacaftor tablet, film coated |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| ORKAMBI
lumacaftor and ivacaftor tablet, film coated |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| ORKAMBI
lumacaftor and ivacaftor granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ORKAMBI
lumacaftor and ivacaftor granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| ORKAMBI
lumacaftor and ivacaftor granule |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Vertex Pharmaceuticals Incorporated (602478257) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Vertex Pharmaceuticals Incorporated | 602478257 | MANUFACTURE(51167-809) , ANALYSIS(51167-809, 51167-700, 51167-900, 51167-500, 51167-122) | |