Drug Detail:Orladeyo (Berotralstat [ ber-oh-tral-stat ])
Drug Class: Hereditary angioedema agents
Highlights of Prescribing Information
ORLADEYO (berotralstat) capsules, for oral use
Initial U.S. Approval: 2020
Indications and Usage for Orladeyo
ORLADEYO is a plasma kallikrein inhibitor indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and pediatric patients 12 years and older. (1)
Limitations of Use:
ORLADEYO should not be used for treatment of acute HAE attacks. (1)
Orladeyo Dosage and Administration
- Recommended Dosage: One capsule (150 mg) taken orally once daily with food. (2.1)
See Full Prescribing Information for:
- Dosage adjustment in patients with moderate or severe hepatic impairment. (2.2)
- Dosage adjustment in patients with chronic administration of P-gp or BCRP inhibitors. (2.3)
- Dosage adjustment in patients with persistent gastrointestinal reactions. (2.4)
Dosage Forms and Strengths
Capsules: 150 mg, 110 mg (3)
Contraindications
None (4)
Warnings and Precautions
An increase in QT prolongation can occur at dosages higher than the recommended 150 mg once daily dosage. Additional doses or doses of ORLADEYO higher than 150 mg once daily are not recommended. (5.1)
Adverse Reactions/Side Effects
Most common adverse reactions (≥10%) are abdominal pain, vomiting, diarrhea, back pain, and gastroesophageal reflux disease. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact BioCryst Pharmaceuticals, Inc. at 1-833-633-2279 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
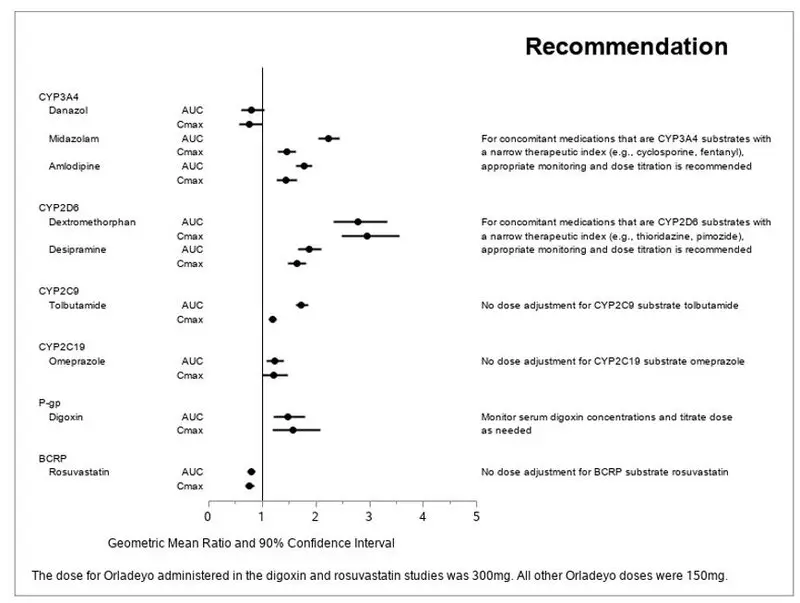
P-gp or BCRP inhibitors: Reduce ORLADEYO dosage when co-administered. (7.1, 12.3)
P-gp inducers: Avoid use with ORLADEYO. (7.1)
CYP2D6, CYP3A4 or P-gp Substrates: Appropriately monitor or dose titrate narrow therapeutic index drugs that are predominantly metabolized by CYP2D6, CYP3A4 or are P-gp substrates when co-administered with ORLADEYO. (7.2, 12.3)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 3/2022
Related/similar drugs
Firazyr, Haegarda, Cinryze, Berinert, Ruconest, KalbitorFull Prescribing Information
1. Indications and Usage for Orladeyo
ORLADEYO® is indicated for prophylaxis to prevent attacks of hereditary angioedema (HAE) in adults and pediatric patients 12 years of age and older.
Limitations of Use:
The safety and effectiveness of ORLADEYO for the treatment of acute HAE attacks have not been established. ORLADEYO should not be used for treatment of acute HAE attacks. Additional doses or doses of ORLADEYO higher than 150 mg once daily are not recommended due to the potential for QT prolongation [see Warnings and Precautions (5.1)].
2. Orladeyo Dosage and Administration
2.1 Recommended Dosage
The recommended dosage of ORLADEYO is one 150 mg capsule taken orally once daily with food.
2.2 Recommended Dosage in Patients with Hepatic Impairment
No dosage adjustment of ORLADEYO is recommended for patients with mild hepatic impairment (Child-Pugh Class A) [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
In patients with moderate or severe hepatic impairment (Child-Pugh B or C), the recommended dosage of ORLADEYO is one 110 mg capsule taken orally once daily with food [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.3 Recommended Dosage for Concomitant Use with P-gp or BCRP Inhibitors
In patients with chronic administration of P-gp or BCRP inhibitors (e.g., cyclosporine), the recommended dosage of ORLADEYO is one 110 mg capsule taken orally once daily with food [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
5. Warnings and Precautions
5.1 Risk of QT Prolongation with Higher-Than-Recommended Dosages
ORLADEYO should not be used for treatment of acute attacks of HAE. Additional doses or doses of ORLADEYO higher than 150 mg once daily are not recommended. An increase in QT was observed at dosages higher than the recommended 150 mg once daily dosage and was concentration dependent [see Clinical Pharmacology (12.2)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reaction is described elsewhere in the labeling:
- QT Prolongation [see Warnings and Precautions (5.1)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
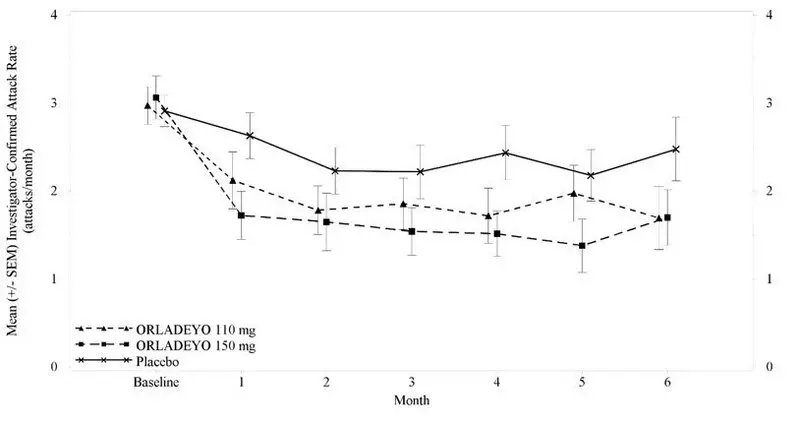
The safety of ORLADEYO is primarily based on 24-week (Part 1) data from a 3-part, double-blind, parallel-group, placebo-controlled study (Trial 1) in 120 patients with Type I or II HAE randomized and dosed with either ORLADEYO 110 mg, 150 mg or placebo, once daily with food. After Week 24, patients who continued in the study received active treatment through 48 weeks.
In Trial 1, a total of 81 patients aged 12 years and older with HAE received at least one dose of ORLADEYO in Part 1. Overall, 66% of patients were female and 93% of patients were Caucasian with a mean age of 41.6 years. The proportion of patients who discontinued study drug prematurely due to adverse reactions was 7% and 3% for patients treated with 110 mg and 150 mg ORLADEYO, respectively, and 3% for placebo-treated patients. No deaths occurred in the trial.
The safety profile of ORLADEYO was generally similar across all subgroups of patients, including analysis by age, sex, and geographic region.
Table 1 shows adverse reactions occurring in ≥10% of patients in any ORLADEYO treatment group that also occurred at a higher rate than in the placebo treatment group in Trial 1.
| Adverse Reaction | Placebo (N=39) | ORLADEYO | ||
|---|---|---|---|---|
| 110 mg (N=41) | 150 mg (N=40) | Total (N=81) |
||
| n (%) | n (%) | n (%) | n (%) | |
|
||||
| Abdominal Pain* | 4 (10) | 4 (10) | 9 (23) | 13 (16) |
| Vomiting | 1 (3) | 4 (10) | 6 (15) | 10 (12) |
| Diarrhea† | 0 | 4 (10) | 6 (15) | 10 (12) |
| Back Pain | 1 (3) | 1 (2) | 4 (10) | 5 (6) |
| Gastroesophageal Reflux Disease | 0 | 4 (10) | 2 (5) | 6 (7) |
Gastrointestinal reactions, including abdominal pain, vomiting, and diarrhea occurred more frequently in patients receiving ORLADEYO 150 mg versus ORLADEYO 110 mg or placebo. These reactions generally occurred early after initiation of treatment with ORLADEYO, became less frequent with time, and typically self-resolved. No patients in the ORLADEYO 150 mg dose group and 1 patient in the ORLADEYO 110 mg dose group discontinued treatment due to a gastrointestinal adverse reaction.
7. Drug Interactions
This section describes clinically relevant drug interactions with ORLADEYO. Drug interaction studies are described elsewhere in the labeling [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of ORLADEYO for prophylaxis to prevent attacks of hereditary angioedema have been established in pediatric patients aged 12 and older. Use of ORLADEYO in this population is supported by evidence from an adequate and well-controlled study (Trial 1) that included adults and a total of 6 adolescent patients aged 12 to <18 years of age. The safety profile and attack rate on study were similar to those observed in adults [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)]. An additional 10 adolescent patients aged 12 to <18 years were enrolled in the open-label study (Trial 2).
The safety and effectiveness of ORLADEYO in pediatric patients <12 years of age have not been established.
8.5 Geriatric Use
The safety and effectiveness of ORLADEYO were evaluated in a subgroup of patients (N=9) aged ≥65 years in Trial 1. Results of the subgroup analysis by age were consistent with overall study results. The safety profile from an additional 5 elderly patients aged ≥65 years enrolled in the open-label, long-term safety study (Trial 2) was consistent with data from Trial 1 [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14)].
8.6 Renal Impairment
No dosage adjustment of ORLADEYO is recommended for patients with mild, moderate or severe renal impairment [see Clinical Pharmacology (12.3)].
ORLADEYO has not been studied in patients with End-Stage Renal Disease (CLCR <15 mL/min or eGFR <15 mL/min/1.73 m2 or patients requiring hemodialysis), and therefore is not recommended for use in these patient populations [see Clinical Pharmacology (12.3)].
8.7 Hepatic Impairment
No dosage adjustment of ORLADEYO is recommended for patients with mild hepatic impairment (Child-Pugh Class A) [see Clinical Pharmacology (12.3)].
In patients with moderate or severe hepatic impairment (Child-Pugh B or C), the recommended dose of ORLADEYO is 110 mg once daily with food [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
11. Orladeyo Description
ORLADEYO (berotralstat) capsules is a plasma kallikrein inhibitor. Berotralstat is presented as the dihydrochloride salt with the chemical name 1-[3-(aminomethyl)phenyl]-N-(5-{(R)-(3-cyanophenyl)[(cyclopropylmethyl)amino]methyl}-2-fluorophenyl)-3-(trifluoromethyl)-1H-pyrazole-5-carboxamide dihydrochloride. The chemical structure is:
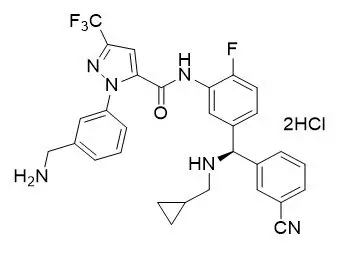
Berotralstat dihydrochloride is a white to off-white powder that is soluble in water at pH ≤4. The molecular formula is C30H26F4N6O ∙ 2HCl and the molecular weight is 635.49 (dihydrochloride).
ORLADEYO is supplied as 150 mg (equivalent to 169.4 mg berotralstat dihydrochloride) and 110 mg (equivalent to 124.3 mg berotralstat dihydrochloride) hard gelatin capsules for oral administration. Each capsule contains the active ingredient berotralstat dihydrochloride and the inactive ingredients colloidal silicon dioxide, crospovidone, magnesium stearate, and pregelatinized starch.
12. Orladeyo - Clinical Pharmacology
12.1 Mechanism of Action
Berotralstat is a plasma kallikrein inhibitor that binds to plasma kallikrein and inhibits its proteolytic activity. Plasma kallikrein is a protease that cleaves high-molecular-weight-kininogen (HMWK) to generate cleaved HMWK (cHMWK) and bradykinin, a potent vasodilator that increases vascular permeability resulting in swelling and pain associated with HAE. In patients with HAE due to C1-inhibitor (C1-INH) deficiency or dysfunction, normal regulation of plasma kallikrein activity is not present, which leads to uncontrolled increases in plasma kallikrein activity and results in angioedema attacks. Berotralstat decreases plasma kallikrein activity to control excess bradykinin generation in patients with HAE.
12.2 Pharmacodynamics
Concentration-dependent inhibition of plasma kallikrein, measured as a reduction from baseline of specific enzyme activity, was demonstrated after oral administration of ORLADEYO once daily in patients with HAE.
12.3 Pharmacokinetics
Following oral administration of berotralstat 150 mg once daily, the steady state Cmax and area under the curve over the dosing interval (AUCtau) are 158 ng/mL (range: 110 to 234 ng/mL) and 2770 ng*hr/mL (range: 1880 to 3790 ng*hr/mL), respectively. Following oral administration of berotralstat 110 mg once daily, the steady-state Cmax and AUCtau are 97.8 ng/mL (range: 63 to 235 ng/mL) and 1600 ng*hr/mL (range: 950 to 4170 ng*hr/mL), respectively.
Berotralstat exposure (Cmax and AUC) increases greater than proportionally with dose and steady state is reached by days 6 to 12. After once-daily administration, exposure of berotralstat at steady state is approximately 5 times that after a single dose.
The pharmacokinetics of berotralstat are similar between healthy adult subjects and in patients with HAE.
16. How is Orladeyo supplied
ORLADEYO (berotralstat) capsules:
- 150 mg: a white opaque body with a black imprint "150" and a light blue opaque cap with a black imprint "BCX".
- 110 mg: light blue opaque capsules with a white imprint "110" on body and a white imprint "BCX" on cap.
- A 28-day supply of ORLADEYO is provided in a carton containing four child-resistant shellpaks, each containing a 7-capsule blister card. NDC 72769-101-01 (150 mg) and NDC 72769-102-01 (110 mg).
- Each carton contains a tamper evident seal.
- Do not use if tamper evident seal is broken or missing.
| ORLADEYO
berotralstat hydrochloride capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| ORLADEYO
berotralstat hydrochloride capsule |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - BioCryst Pharmaceuticals Inc. (618194609) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Cambrex Charles City | 782974257 | API MANUFACTURE(72769-101, 72769-102) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent CTS, LLC | 962674474 | MANUFACTURE(72769-101, 72769-102) , ANALYSIS(72769-101, 72769-102) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Catalent Pharma Solutions LLC | 014904112 | PACK(72769-101, 72769-102) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Pharmaceuticals, Inc. | 005286822 | MANUFACTURE(72769-101, 72769-102) , ANALYSIS(72769-101, 72769-102) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| AndersonBrecon Inc. A PCI Pharma Services Company | 053217022 | PACK(72769-101, 72769-102) , LABEL(72769-101, 72769-102) | |