Drug Detail:Oseni (Alogliptin and pioglitazone [ al-oh-glip-tin-and-pye-oh-gli-ta-zone ])
Drug Class: Antidiabetic combinations
Highlights of Prescribing Information
OSENI (alogliptin and pioglitazone) tablets, for oral use
Initial U.S. Approval: 2013
WARNING: CONGESTIVE HEART FAILURE
See full prescribing information for complete boxed warning
- Thiazolidinediones, including pioglitazone, cause or exacerbate congestive heart failure in some patients. (5.1)
- After initiation of OSENI and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone in OSENI must be considered. (5.1)
- OSENI is not recommended in patients with symptomatic heart failure. (5.1)
- Initiation of OSENI in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated. (4, 5.1)
Indications and Usage for Oseni
OSENI is a combination of alogliptin, a dipeptidyl peptidase-4 inhibitor, and pioglitazone, a thiazolidinedione, indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus. (1)
Limitations of Use: Should not be used in patients with type 1 diabetes mellitus. (1)
Oseni Dosage and Administration
- Individualize the starting dose of OSENI based on the patient's current regimen and concurrent medical condition but do not exceed a daily dose of alogliptin 25 mg and pioglitazone 45 mg. (2.1)
- Can be taken with or without food. (2.1)
- Limit initial dose of pioglitazone to 15 mg once daily in patients with NYHA Class I or II heart failure. (2.1)
- Adjust dose if moderate renal impairment. (2.2)
| Degree of Renal Impairment | Creatinine Clearance (mL/min) | Recommended Dosing |
|---|---|---|
| Moderate | ≥30 to <60 | 12.5 mg/15 mg, 12.5 mg/30 mg or 12.5 mg/45 mg once daily |
- OSENI is not recommended for patients with severe renal impairment or end-stage renal disease (ESRD) requiring dialysis. (2.2)
- The maximum recommended dose of pioglitazone is 15 mg once daily in patients taking strong CYP2C8 inhibitors (e.g., gemfibrozil). (2.3, 7.1)
Dosage Forms and Strengths
Tablets:
25 mg alogliptin and 15 mg pioglitazone, 25 mg alogliptin and 30 mg pioglitazone, 25 mg alogliptin and 45 mg pioglitazone. (3)
12.5 mg alogliptin and 15 mg pioglitazone, 12.5 mg alogliptin and 30 mg pioglitazone, 12.5 mg alogliptin and 45 mg pioglitazone. (3)
Contraindications
- Serious hypersensitivity reaction to alogliptin or pioglitazone, components of OSENI, or any of the excipients. (4)
- Do not initiate OSENI in patients with established NYHA Class III or IV heart failure. (4)
Warnings and Precautions
- Congestive heart failure: Fluid retention may occur and can exacerbate or lead to congestive heart failure. Combination use with insulin and use in congestive heart failure NYHA Class I and II may increase risk. Consider the risks and benefits of OSENI prior to initiating treatment in patients at risk for heart failure. Monitor patients at risk for heart failure for signs and symptoms. If heart failure develops, evaluate and manage according to current standards of care and consider discontinuation of OSENI. (5.1)
- Pancreatitis: There have been postmarketing reports of acute pancreatitis. If pancreatitis is suspected, promptly discontinue OSENI. (5.2)
- Hypersensitivity: There have been postmarketing reports of serious hypersensitivity reactions in patients treated with alogliptin such as anaphylaxis, angioedema and severe cutaneous adverse reactions, including Stevens-Johnson syndrome. In such cases, promptly discontinue OSENI, assess for other potential causes, institute appropriate monitoring and treatment and initiate alternative treatment for diabetes. (5.3)
- Hepatic effects: Postmarketing reports of hepatic failure, sometimes fatal. Causality cannot be excluded. If liver injury is detected, promptly interrupt OSENI and assess patient for probable cause, then treat cause if possible, to resolution or stabilization. Do not restart OSENI if liver injury is confirmed and no alternative etiology can be found. Use with caution in patients with liver disease. (5.4)
- Edema: Dose-related edema may occur. (5.5)
- Fractures: Increased incidence in female patients. Apply current standards of care for assessing and maintaining bone health. (5.6)
- Bladder cancer: May increase the risk of bladder cancer. Do not use in patients with active bladder cancer. Use caution when using in patients with a prior history of bladder cancer. (5.7)
- Hypoglycemia: When an insulin secretagogue (e.g., sulfonylurea) or insulin is used in combination with OSENI, a lower dose of insulin secretagogue or insulin may be required to minimize the risk of hypoglycemia. (5.8)
- Macular edema: Postmarketing reports. Recommend regular eye exams in all patients with diabetes according to current standards of care with prompt evaluation for acute visual changes. (5.9)
- Arthralgia: Severe and disabling arthralgia has been reported in patients taking DPP-4 inhibitors. Consider as a possible cause for severe joint pain and discontinue if appropriate. (5.10)
- Bullous pemphigoid: There have been postmarketing reports of bullous pemphigoid requiring hospitalization in patients taking DPP-4 inhibitors. Tell patients to report development of blisters or erosions. If bullous pemphigoid is suspected, discontinue OSENI. (5.11)
Adverse Reactions/Side Effects
The most common adverse reactions (4% or greater incidence) are nasopharyngitis, back pain and upper respiratory tract infection. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals America, Inc. at 1-877-TAKEDA-7 (1-877-825-3327) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong CYP2C8 inhibitors (e.g., gemfibrozil) increase pioglitazone concentrations. Limit the pioglitazone dose to 15 mg daily. (2.3, 7.1)
- CYP2C8 inducers (e.g., rifampin) may decrease pioglitazone concentrations. (7.2)
- Topiramate may decrease pioglitazone concentrations. (7.3)
Use In Specific Populations
- Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy. (8.3)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 3/2022
Related/similar drugs
Mounjaro, metformin, Trulicity, Lantus, Victoza, Levemir, TresibaFull Prescribing Information
WARNING: CONGESTIVE HEART FAILURE
- Thiazolidinediones, including pioglitazone, which is a component of OSENI, cause or exacerbate congestive heart failure in some patients [see Warnings and Precautions (5.1)].
- After initiation of OSENI and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone in OSENI must be considered [see Warnings and Precautions (5.1)].
- OSENI is not recommended in patients with symptomatic heart failure [see Warnings and Precautions (5.1)].
- Initiation of OSENI in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated [see Contraindications (4) and Warnings and Precautions (5.1)].
1. Indications and Usage for Oseni
OSENI is indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus.
2. Oseni Dosage and Administration
2.1 Recommendations for All Patients
OSENI should be taken once daily and can be taken with or without food. Do not spilt tablets.
The recommended starting dose for OSENI (alogliptin and pioglitazone):
- for patients inadequately controlled on diet and exercise is 25 mg/15 mg or 25 mg/30 mg,
- for patients inadequately controlled on metformin monotherapy is 25 mg/15 mg or 25 mg/30 mg,
- for patients on alogliptin who require additional glycemic control is 25 mg/15 mg or 25 mg/30 mg,
- for patients on pioglitazone who require additional glycemic control is 25 mg/15 mg, 25 mg/30 mg or 25 mg/45 mg as appropriate based upon current therapy,
- for patients switching from alogliptin coadministered with pioglitazone, OSENI may be initiated at the dose of alogliptin and pioglitazone based upon current therapy,
- for patients with congestive heart failure (NYHA Class I or II) is 25 mg/15 mg.
The OSENI dose can be titrated up to a maximum of 25 mg/45 mg once daily based on glycemic response as determined by hemoglobin A1c (A1C).
After initiation of OSENI or with dose increase, monitor patients carefully for adverse reactions related to fluid retention as has been seen with pioglitazone (e.g., weight gain, edema and signs and symptoms of congestive heart failure) [see Boxed Warning and Warnings and Precautions (5.1)].
2.2 Patients with Renal Impairment
No dose adjustment of OSENI is necessary for patients with mild renal impairment (creatinine clearance [CrCl] ≥60 mL/min).
The dose of OSENI is 12.5 mg/15 mg, 12.5 mg/30 mg or 12.5 mg/45 mg once daily for patients with moderate renal impairment (CrCl ≥30 to <60 mL/min).
OSENI is not recommended for patients with severe renal impairment or ESRD [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)]. Coadministration of pioglitazone and alogliptin 6.25 mg once daily based on individual requirements may be considered in these patients.
Because there is a need for dose adjustment based upon renal function, assessment of renal function is recommended prior to initiation of OSENI therapy and periodically thereafter.
2.3 Coadministration with Strong CYP2C8 Inhibitors
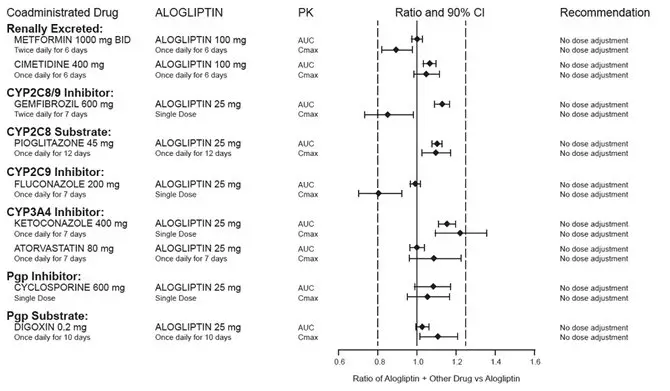
Coadministration of pioglitazone and gemfibrozil, a strong CYP2C8 inhibitor, increases pioglitazone exposure approximately three-fold. Therefore, the maximum recommended dose of OSENI is 25 mg/15 mg daily when used in combination with gemfibrozil or other strong CYP2C8 inhibitors [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
| Pharmaceutical form | Strength (alogliptin/ pioglitazone) | Color | Shape | Markings (on one side) |
|---|---|---|---|---|
| film-coated tablets | 12.5 mg/15 mg | Pale yellow | Round biconvex | "A/P" and 12.5/15" |
| film-coated tablets | 12.5 mg/30 mg | Pale peach | Round biconvex | "A/P and "12.5/30" |
| film-coated tablets | 12.5 mg/45 mg | Pale red | Round biconvex | "A/P" and 12.5/45" |
| film-coated tablets | 25 mg/15 mg | Yellow | Round biconvex | "A/P" and "25/15" |
| film-coated tablets | 25 mg/30 mg | Peach | Round biconvex | "A/P" and "25/30" |
| film-coated tablets | 25 mg/45 mg | Red | Round biconvex | "A/P" and "25/45" |
4. Contraindications
Serious hypersensitivity reaction to alogliptin or pioglitazone or any of the excipients in OSENI, such as anaphylaxis, angioedema and severe cutaneous adverse reactions have been reported [see Warnings and Precautions (5.3), Adverse reactions (6.2)].
Do not initiate in patients with NYHA Class III or IV heart failure [see Boxed Warning].
5. Warnings and Precautions
5.1 Congestive Heart Failure
Consider the risks and benefits of OSENI prior to initiating treatment in patients at risk for heart failure, such as those with a prior history of heart failure and a history of renal impairment, and observe these patients for signs and symptoms of congestive heart failure. Patients should be advised of the characteristic symptoms of congestive heart failure and should be instructed to immediately report such symptoms. If congestive heart failure develops, it should be managed according to current standards of care and consider discontinuation of OSENI.
5.2 Pancreatitis
Acute pancreatitis has been reported in the postmarketing setting and in randomized clinical trials. In glycemic control trials in patients with type 2 diabetes, acute pancreatitis was reported in six (0.2%) patients treated with alogliptin 25 mg and two (<0.1%) patients treated with active comparators or placebo. In the EXAMINE trial (a cardiovascular outcomes trial of patients with type 2 diabetes and high cardiovascular (CV) risk), acute pancreatitis was reported in ten (0.4%) patients treated with alogliptin and in seven (0.3%) patients treated with placebo.
It is unknown whether patients with a history of pancreatitis are at increased risk for pancreatitis while using OSENI.
After initiation of OSENI, patients should be observed for signs and symptoms of pancreatitis. If pancreatitis is suspected, OSENI should promptly be discontinued and appropriate management should be initiated.
5.3 Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions in patients treated with alogliptin. These reactions include anaphylaxis, angioedema and severe cutaneous adverse reactions, including Stevens-Johnson syndrome. If a serious hypersensitivity reaction is suspected, discontinue OSENI, assess for other potential causes for the event and institute alternative treatment for diabetes [see Adverse Reactions (6.2)]. Use caution in patients with a history of angioedema with another dipeptidyl peptidase-4 (DPP-4) inhibitor because it is unknown whether such patients will be predisposed to angioedema with OSENI.
5.4 Hepatic Effects
There have been postmarketing reports of fatal and nonfatal hepatic failure in patients taking pioglitazone or alogliptin, although some of the reports contain insufficient information necessary to establish the probable cause [see Adverse Reactions (6.2)].
In glycemic control trials of alogliptin in patients with type 2 diabetes, serum alanine aminotransferase (ALT) elevations greater than three times the upper limit of normal (ULN) were reported in 1.3% of patients treated with alogliptin 25 mg and 1.7% of patients treated with active comparators or placebo. In the EXAMINE trial (a cardiovascular outcomes trial of patients with type 2 diabetes and high cardiovascular (CV) risk), increases in serum alanine aminotransferase three times the upper limit of the reference range occurred in 2.4% of patients treated with alogliptin and in 1.8% of patients treated with placebo.
Patients with type 2 diabetes may have fatty liver disease or cardiac disease with episodic congestive heart failure, both of which may cause liver test abnormalities, and they may also have other forms of liver disease, many of which can be treated or managed. Therefore, obtaining a liver test panel (ALT, aspartate aminotransferase [AST], alkaline phosphatase and total bilirubin) and assessing the patient is recommended before initiating OSENI therapy. In patients with abnormal liver tests, OSENI should be initiated with caution.
Measure liver tests promptly in patients who report symptoms that may indicate liver injury, including fatigue, anorexia, right upper abdominal discomfort, dark urine or jaundice. In this clinical context, if the patient is found to have abnormal liver tests (ALT greater than three times the upper limit of the reference range), OSENI treatment should be interrupted and an investigation done to establish the probable cause. OSENI should not be restarted in these patients without another explanation for the liver test abnormalities.
5.8 Hypoglycemia with Concomitant Use with Insulin or Insulin Secretagogues
Insulin and insulin secretagogues, such as sulfonylureas, are known to cause hypoglycemia. Therefore, a lower dose of insulin or insulin secretagogue may be required to minimize the risk of hypoglycemia when used in combination with OSENI.
5.10 Severe and Disabling Arthralgia
There have been postmarketing reports of severe and disabling arthralgia in patients taking DPP-4 inhibitors. The time to onset of symptoms following initiation of drug therapy varied from one day to years. Patients experienced relief of symptoms upon discontinuation of the medication. A subset of patients experienced a recurrence of symptoms when restarting the same drug or a different DPP-4 inhibitor. Consider DPP-4 inhibitors as a possible cause for severe joint pain and discontinue drug if appropriate.
5.11 Bullous Pemphigoid
Postmarketing cases of bullous pemphigoid requiring hospitalization have been reported with DPP-4 inhibitor use. In reported cases, patients typically recovered with topical or systemic immunosuppressive treatment and discontinuation of the DPP-4 inhibitor. Tell patients to report development of blisters or erosions while receiving OSENI. If bullous pemphigoid is suspected, OSENI should be discontinued and referral to a dermatologist should be considered for diagnosis and appropriate treatment.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described below or elsewhere in the prescribing information:
- Congestive Heart Failure [see Warnings and Precautions (5.1)]
- Pancreatitis [see Warnings and Precautions (5.2)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Hepatic Effects [see Warnings and Precautions (5.4)]
- Severe and Disabling Arthralgia [see Warnings and Precautions (5.10)]
- Bullous Pemphigoid [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Pioglitazone
Over 8500 patients with type 2 diabetes have been treated with pioglitazone in randomized, double-blind, controlled clinical trials, including 2605 patients with type 2 diabetes and macrovascular disease treated with pioglitazone in the PROactive clinical trial. In these trials, over 6000 patients have been treated with pioglitazone for six months or longer, over 4500 patients have been treated with pioglitazone for one year or longer, and over 3000 patients have been treated with pioglitazone for at least two years.
8. Use In Specific Populations
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as therapy with pioglitazone, like other thiazolidinediones, may result in ovulation in some anovulatory women.
8.4 Pediatric Use
Safety and effectiveness of OSENI in pediatric patients have not been established.
OSENI is not recommended for use in pediatric patients based on adverse effects observed in adults, including fluid retention and congestive heart failure, fractures and urinary bladder tumors [see Warnings and Precautions (5.1, 5.5, 5.6, 5.7)].
11. Oseni Description
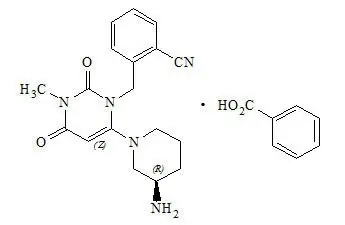
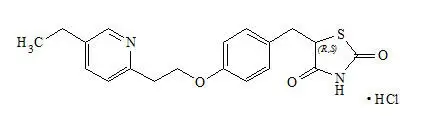
OSENI tablets contain two oral antihyperglycemic drugs used in the management of type 2 diabetes: alogliptin and pioglitazone.
12. Oseni - Clinical Pharmacology
12.1 Mechanism of Action
OSENI combines two antihyperglycemic agents with complementary and distinct mechanisms of action to improve glycemic control in patients with type 2 diabetes: alogliptin, a selective inhibitor of DPP-4, and pioglitazone, a member of the TZD class.
12.3 Pharmacokinetics
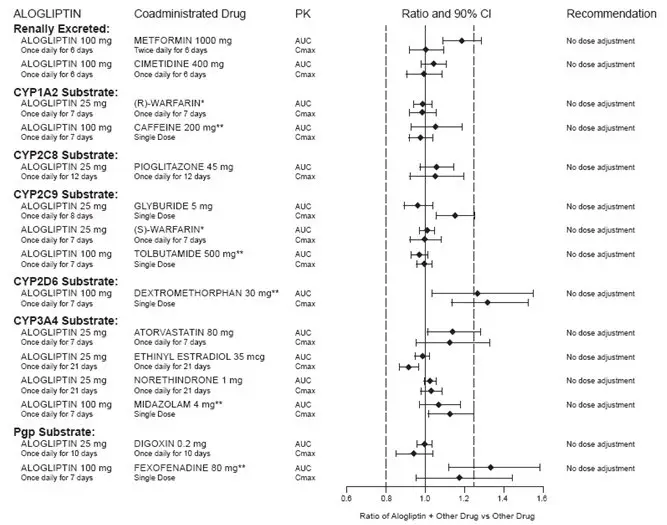
Drug Interactions
Coadministration of alogliptin 25 mg once daily with a CYP2C8 substrate, pioglitazone 45 mg once daily for 12 days had no clinically meaningful effects on the pharmacokinetics of pioglitazone and its active metabolites.
Specific pharmacokinetic drug interaction studies with OSENI have not been performed, although such studies have been conducted with the individual components of OSENI (alogliptin and pioglitazone).
13. Nonclinical Toxicology
14. Clinical Studies
The coadministration of alogliptin and pioglitazone has been studied in patients with type 2 diabetes inadequately controlled on either diet and exercise alone or on metformin alone.
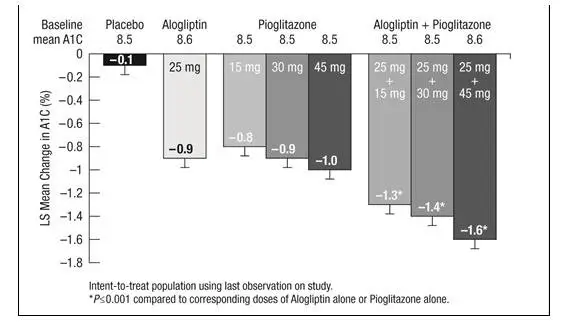
There have been no clinical efficacy studies conducted with OSENI; however, bioequivalence of OSENI with coadministered alogliptin and pioglitazone tablets was demonstrated, and efficacy of the combination of alogliptin and pioglitazone has been demonstrated in four Phase 3 efficacy studies.
In patients with type 2 diabetes, treatment with OSENI produced clinically meaningful and statistically significant improvements in A1C compared to either alogliptin or pioglitazone alone. As is typical for trials of agents to treat type 2 diabetes, the mean reduction in A1C with OSENI appears to be related to the degree of A1C elevation at baseline.
16. How is Oseni supplied
OSENI tablets are available in the following strengths and packages:
25 mg/15 mg tablet: yellow, round, biconvex and film-coated with both "A/P" and "25/15" printed on one side, available in:
|
NDC 64764-251-03 |
Bottles of 30 tablets |
|
NDC 64764-251-04 |
Bottles of 90 tablets |
|
NDC 64764-251-05 |
Bottles of 500 tablets |
25 mg/30 mg tablet: peach, round, biconvex and film-coated with both "A/P" and "25/30" printed on one side, available in:
|
NDC 64764-253-03 |
Bottles of 30 tablets |
|
NDC 64764-253-04 |
Bottles of 90 tablets |
|
NDC 64764-253-05 |
Bottles of 500 tablets |
25 mg/45 mg tablet: red, round, biconvex, film-coated and with both "A/P" and "25/45" printed on one side, available in:
|
NDC 64764-254-03 |
Bottles of 30 tablets |
|
NDC 64764-254-04 |
Bottles of 90 tablets |
|
NDC 64764-254-05 |
Bottles of 500 tablets |
12.5 mg/15 mg tablet: pale yellow, round, biconvex and film-coated with both "A/P" and "12.5/15" printed on one side, available in:
|
NDC 64764-121-03 |
Bottles of 30 tablets |
|
NDC 64764-121-04 |
Bottles of 90 tablets |
|
NDC 64764-121-05 |
Bottles of 500 tablets |
12.5 mg/30 mg tablet: pale peach, round, biconvex and film-coated with both "A/P" and "12.5/30" printed on one side, available in:
|
NDC 64764-123-03 |
Bottles of 30 tablets |
|
NDC 64764-123-04 |
Bottles of 90 tablets |
|
NDC 64764-123-05 |
Bottles of 500 tablets |
12.5 mg/45 mg tablet: pale red, round, biconvex and film-coated with both "A/P" and "12.5/45" printed on one side, available in:
|
NDC 64764-124-03 |
Bottles of 30 tablets |
|
NDC 64764-124-04 |
Bottles of 90 tablets |
|
NDC 64764-124-05 |
Bottles of 500 tablets |
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| MEDICATION GUIDE OSENI (OH-senn-ee) (alogliptin and pioglitazone) tablets |
|||||
|---|---|---|---|---|---|
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | 03/2022 | ||||
| Read this Medication Guide carefully before you start taking OSENI and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about OSENI, ask your doctor or pharmacist. | |||||
| What is the most important information I should know about OSENI? OSENI can cause serious side effects, including:
Tell your doctor if you have ever had: |
|||||
|
|
|
|||
| Stop taking OSENI and call your doctor right away if you have pain in your stomach area (abdomen) that is severe and will not go away. The pain may be felt going from your abdomen through to your back. The pain may happen with or without vomiting. These may be symptoms of pancreatitis. | |||||
What is OSENI?
|
|||||
| Who should not take OSENI? Do not take OSENI if you:
|
|||||
|
|
||||
| If you have these symptoms, stop taking OSENI and contact your doctor or go to the nearest hospital emergency room right away. | |||||
| What should I tell my doctor before and during treatment with OSENI?
Before you start taking OSENI, tell your doctor if you:
Know the medicines you take. Keep a list of them and show it to your doctor and pharmacist before you start a new medicine. OSENI may affect the way other medicines work, and other medicines may affect how OSENI works. Contact your doctor before you start or stop other types of medicines. |
|||||
How should I take OSENI?
|
|||||
| What are the possible side effects of OSENI? OSENI can cause serious side effects, including:
|
|||||
|
|
||||
If you have these symptoms, stop taking OSENI and contact your doctor right away or go to the nearest hospital emergency room
|
|||||
|
|
|
|||
|
|||||
|
|
|
|||
|
|||||
|
|
|
|
|
|
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the possible side effects of OSENI. For more information, ask your doctor or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||||
How should I store OSENI?
|
|||||
| General information about the safe and effective use of OSENI
Medicines are sometimes prescribed for purposes other than those listed in the Medication Guide. Do not take OSENI for a condition for which it was not prescribed. Do not give OSENI to other people, even if they have the same symptoms you have. It may harm them. This Medication Guide summarizes the most important information about OSENI. If you would like to know more information, talk with your doctor. You can ask your doctor or pharmacist for information about OSENI that is written for health professionals. For more information, go to www.oseni.com or call 1-877-TAKEDA-7 (1-877-825-3327). ALP008 R11 |
|||||
| OSENI
alogliptin and pioglitazone tablet, film coated |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| OSENI
alogliptin and pioglitazone tablet, film coated |
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||
| OSENI
alogliptin and pioglitazone tablet, film coated |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| OSENI
alogliptin and pioglitazone tablet, film coated |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| OSENI
alogliptin and pioglitazone tablet, film coated |
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||
| OSENI
alogliptin and pioglitazone tablet, film coated |
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||
| Labeler - Takeda Pharmaceuticals America, Inc. (039997266) |