Drug Detail:Otiprio (Ciprofloxacin otic [ sip-roe-flox-a-sin-oh-tik ])
Drug Class: Otic anti-infectives
Highlights of Prescribing Information
OTIPRIO® (ciprofloxacin otic suspension), for intratympanic or otic use
Initial U.S. Approval: 1987
Recent Major Changes
| Indications and Usage (1) | 3/2018 |
| Dosage and Administration (2) | 3/2018 |
Indications and Usage for Otiprio Ear Drops
OTIPRIO is a fluoroquinolone antibacterial indicated for the following conditions:
- The treatment of pediatric patients (age 6 months and older) with bilateral otitis media with effusion undergoing tympanostomy tube placement. (1)
- The treatment of acute otitis externa in patients 6 months of age and older due to Pseudomonas aeruginosa and Staphylococcus aureus. (1)
Otiprio Ear Drops Dosage and Administration
- OTIPRIO is for intratympanic or otic administration by a healthcare professional only. (2.1)
- OTIPRIO is intended for single-patient use with up to two doses available in each vial. (2.1)
- For bilateral otitis media with effusion, administer OTIPRIO as a single intratympanic administration of one 0.1 mL (6 mg) dose into each affected ear, following suctioning of the middle ear effusion. (2.1)
- For acute otitis externa, administer OTIPRIO as a single 0.2 mL (12 mg) administration to the affected ear(s). (2.1)
- See Full Prescribing Information for directions for OTIPRIO dose preparation. (2.2)
Dosage Forms and Strengths
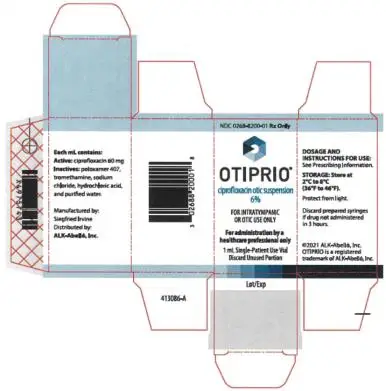
Otic Suspension: Each OTIPRIO vial contains 1 mL of 6% (60 mg/mL) ciprofloxacin otic suspension. (3)
Contraindications
OTIPRIO is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to quinolones, or to any component of OTIPRIO. (4)
Warnings and Precautions
Potential for Microbial Overgrowth: OTIPRIO may result in overgrowth of non-susceptible bacteria and fungi. (5.1)
Adverse Reactions/Side Effects
Otitis Media with Effusion: The most frequently occurring adverse reactions (with an incidence rate greater than 3%) were nasopharyngitis and irritability. (6.1)
Acute Otitis Externa: The most frequently occurring adverse reactions (with an incidence rate of at least 2%) were: ear pruritus, headache, otitis media and ear discomfort. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact ALK-Abelló, Inc. at 1-855-216-6497 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 6/2021
Full Prescribing Information
1. Indications and Usage for Otiprio Ear Drops
OTIPRIO is indicated for
● The treatment of pediatric patients (6 months of age and older) with bilateral otitis media with effusion undergoing tympanostomy tube placement.
● The treatment of acute otitis externa in patients 6 months of age and older due to Pseudomonas aeruginosa and Staphylococcus aureus.
2. Otiprio Ear Drops Dosage and Administration
2.1 Dosage and Important Administration Instructions
● OTIPRIO is for intratympanic or otic administration by a healthcare professional only.
● OTIPRIO is intended for single-patient use, discard unused portion.
● For bilateral otitis media with effusion, administer OTIPRIO as a single intratympanic administration of one 0.1 mL (6 mg) dose into each affected ear of pediatric patients (6 months of age and older), following suctioning of middle ear effusion.
● For acute otitis externa, administer OTIPRIO as a single 0.2 mL (12 mg) administration to the external ear canal of each affected ear of patients aged 6 months and older.
3. Dosage Forms and Strengths
Otic Suspension: Each 1 mL of OTIPRIO contains a white, preservative-free, sterile otic suspension consisting of 6% (60 mg/mL) ciprofloxacin in a single-patient use glass vial.
4. Contraindications
OTIPRIO is contraindicated in patients with a history of hypersensitivity to ciprofloxacin, to other quinolones, or to any of the components of OTIPRIO.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
Otitis Media with Effusion
In two randomized, sham-controlled Phase 3 clinical trials, 530 pediatric patients with bilateral otitis media with effusion undergoing tympanostomy tube placement were treated with OTIPRIO or sham administered intratympanically as a single dose (0.1 mL to each ear). The median age of the pediatric patients enrolled in the clinical trials was 1.5 years; 62% of patients were 6 months through 2 years of age and 38% of patients were greater than 2 years of age.
Adverse reactions that occurred in at least 3% of OTIPRIO patients and at an incidence greater than sham are presented in Table 1.
| Adverse Reactions | OTIPRIO
(N=357) | Sham
(N=173) |
| Nasopharyngitis | 5% | 4% |
| Irritability | 5% | 3% |
| Rhinorrhea | 3% | 2% |
Acute Otitis Externa
In a single randomized, sham controlled Phase 3 clinical trial, 259 pediatric and adult patients with acute otitis externa were treated with OTIPRIO or sham administered by a healthcare professional to the external ear canal as a single dose (0.2
mL to each affected ear). The median age of the patients enrolled in the clinical trial was 34 years; 26% were pediatric patients (age 3 to 17 years), 65% were adults (age 18 to 64 years), and 8% were elderly patients (age 65 years and older).
Adverse reactions that occurred in at least 2% of OTIPRIO patients and at an incidence greater than sham are presented in Table 2.
| Adverse Reactions | OTIPRIO
(N=127) | Sham
(N=132) |
| Ear Pruritus | 2% | 2% |
| Headache | 2% | 1% |
| Otitis Media | 2% | 1% |
| Ear Discomfort | 2% | 0% |
12. Otiprio Ear Drops - Clinical Pharmacology
12.1 Mechanism of Action
Ciprofloxacin is a fluoroquinolone antibacterial [see Microbiology (12.4)].
12.4 Microbiology
Mechanism of Action
The bactericidal action of ciprofloxacin results from interference with the enzyme DNA gyrase, which is needed for the synthesis of bacterial DNA.
Resistance
Bacterial resistance to fluoroquinolones can develop through chromosomally- or plasmid-mediated mechanisms. In vitro studies demonstrated cross-resistance between ciprofloxacin and some fluoroquinolones. There is generally no cross- resistance between ciprofloxacin and other classes of antibacterial agents, such as beta-lactams or aminoglycosides.
Antimicrobial Activity
Ciprofloxacin has been shown to be active against most isolates of the following bacteria:
Gram-positive Bacteria
Staphylococcus aureus
Streptococcus pneumoniae
Gram-negative Bacteria
Haemophilus influenzae
Moraxella catarrhalis
Pseudomonas aeruginosa
16. How is Otiprio Ear Drops supplied
OTIPRIO is a sterile, preservative-free, otic suspension of 6% (60 mg/mL, w/v) ciprofloxacin in a neutral pH buffered, isotonic solution containing poloxamer 407.
Each OTIPRIO carton contains 1 mL of 6% (60 mg/mL, w/v) ciprofloxacin in a 2 mL single-patient use glass vial fitted with a stopper not made with natural rubber latex. (NDC 0268-8200-01)
OTIPRIO should be stored at 2 to 8°C (36 to 46°F) until prior to use to prevent thickening during preparation. Protect from light. Store in the original carton until dose preparation.
| OTIPRIO
otiprio suspension |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - ALK-Abello, Inc. (809998847) |