Drug Class: Gonadotropins
Ovidrel Injection - Clinical Pharmacology
The physicochemical, immunological, and biological activities of recombinant hCG are comparable to those of placental and human pregnancy urine-derived hCG. Choriogonadotropin alfa stimulates late follicular maturation and resumption of oocyte meiosis, and initiates rupture of the pre-ovulatory ovarian follicle. Choriogonadotropin alfa, the active component of Ovidrel® PreFilled Syringe , is an analogue of Luteinizing Hormone (LH) and binds to the LH/hCG receptor of the granulosa and theca cells of the ovary to effect these changes in the absence of an endogenous LH surge. In pregnancy, hCG, secreted by the placenta, maintains the viability of the corpus luteum to provide the continued secretion of estrogen and progesterone necessary to support the first trimester of pregnancy. Ovidrel® PreFilled Syringe is administered when monitoring of the patient indicates that sufficient follicular development has occurred in response to FSH treatment for ovulation induction.
Pharmacokinetics
When given by intravenous administration, the pharmacokinetic profile of Ovidrel® followed a biexponential model and was linear over a range of 25 µg to 1000 µg. Pharmacokinetic parameter estimates following SC administration of Ovidrel® 250 µg to females are presented in Table 1.
| Ovidrel® 250 µg SC | |
|---|---|
| Cmax: peak concentration (above baseline), tmax: time of Cmax, AUC: total area under the curve, t½: elimination half-life, F: bioavailability | |
|
|
| Cmax (IU/L) | 121 ± 44 |
| tmax (h)* | 24 (12-24) |
| AUC (h∙IU/L) | 7701 ± 2101 |
| t½ (h) | 29 ± 6 |
| F | 0.4 ± 0.1 |
Bioequivalence of Formulations
Ovidrel® PreFilled Syringe (choriogonadotropin alfa injection) has been determined to be bioequivalent to Ovidrel® (choriogonadotropin alfa for injection) based on the statistical evaluation of AUC and Cmax. A summary of the Ovidrel® PreFilled Syringe pharmacokinetic parameters is presented in Table 2.
| Parameter | Cmax
(mIU/mL) | AUClast
(mIU∙h/mL) | AUC (mIU∙h/mL) | AUCextrapolated
(%) | tmax
(h) |
|---|---|---|---|---|---|
| Abbreviations are: Cmax: peak concentration (above baseline); tmax : time of Cmax | |||||
| Mean | 125 | 10050 | 10350 | 2.85 | 20.0 |
| (Min-Max) | (68.0-294) | (5646-14850) | (5800-15100) | (1.08-6.27) | (9.00-48.0) |
Clinical Studies
The safety and efficacy of Ovidrel® have been examined in three well-controlled studies in women; two studies for assisted reproductive technologies (ART) and one study for ovulation induction (OI).
Assisted Reproductive Technologies (ART)
The safety and efficacy of Ovidrel® 250 µg and Ovidrel® 500 µg administered subcutaneously versus 10,000 USP Units of an approved urinary-derived hCG product administered intramuscularly were assessed in a randomized, open-label, multicenter study in infertile women undergoing in vitro fertilization and embryo transfer (Study 7927). The study was conducted in 20 U.S. centers.
The primary efficacy parameter in this single-cycle study was the number of oocytes retrieved. 297 patients entered the study, of whom 94 were randomized to receive Ovidrel® 250 µg. The number of oocytes retrieved was similar for the Ovidrel® and urinary-derived hCG (10,000 USP Units) treatment groups. The efficacy of Ovidrel® 250 µg and Ovidrel® 500 µg were both found to be clinically and statistically equivalent to that of the approved urinary-derived hCG product and to each other. The efficacy results for the patients who received Ovidrel® 250 µg are summarized in Table 3.
| Parameter | Ovidrel® 250 µg (n = 94) |
|---|---|
|
|
| Mean number of oocytes retrieved per patient | 13.60 |
| Mean number of mature oocytes retrieved per patient | 7.6 |
| Mean number of 2 PN fertilized oocytes per patient | 7.2 |
| Mean number of 2 PN or cleaved embryos per patient | 7.6 |
| Implantation rate per embryo transferred (%) | 18.7 |
| Mean mid-luteal serum progesterone levels (nmol/L*) | 423 |
| Clinical pregnancy rate per initiated treatment cycle (%)† | 35.1 |
| Clinical pregnancy rate per transfer (%)† | 36.3 |
For the 33 patients who achieved a clinical pregnancy with Ovidrel® 250 µg, the outcomes of the pregnancies are presented in Table 4.
| Parameter | Ovidrel® 250 µg (n = 33) |
|---|---|
| Clinical pregnancies not reaching term | 4 (12.1%) |
| Live births | 29 (87.9%) |
| Singleton | 20 (69.0%) |
| Multiple birth | 9 (31.0%) |
The safety and efficacy of Ovidrel® 250 µg administered subcutaneously versus 5,000 IU of an approved urinary-derived hCG product administered subcutaneously were assessed in a second, randomized, multicenter study in infertile women undergoing in vitro fertilization and embryo transfer (Study 7648). This double-blinded study was conducted in nine centers in Europe and Israel.
The primary efficacy parameter in this single-cycle study was the number of oocytes retrieved per patient. 205 patients entered the study, of whom 97 received Ovidrel® 250 µg. The efficacy of Ovidrel® 250 µg was found to be clinically and statistically equivalent to that of the approved urinary-derived hCG product. The results for the 97 patients who received Ovidrel® 250 µg are summarized in Table 5.
| Parameter | Ovidrel® 250 µg (n = 97) |
|---|---|
|
|
| Mean number of oocytes retrieved per patient | 10.6 |
| Mean number of mature oocytes retrieved per patient | 10.1 |
| Mean number of 2 PN fertilized oocytes per patient | 5.7 |
| Mean number of 2 PN or cleaved embryos per patient | 5.1 |
| Implantation rate per embryo transferred (%) | 17.4 |
| Mean mid-luteal serum progesterone levels (nmol/L)* | 394 |
| Clinical pregnancy rate per initiated treatment cycle (%)† | 33 |
| Clinical pregnancy rate per transfer (%)† | 37.6 |
For the 32 patients who achieved a clinical pregnancy with Ovidrel® 250 µg, the outcomes of the pregnancies are presented in Table 6.
| Parameter | Ovidrel® 250 µg (n = 32) |
|---|---|
| Clinical Pregnancies not reaching term | 6 (18.8%) |
| Live births | 26 (81.2%) |
| Singleton | 18 (69.2%) |
| Multiple birth | 8 (30.8%) |
Ovulation Induction (OI)
The safety and efficacy of Ovidrel® 250 µg administered subcutaneously versus 5,000 IU of an approved urinary-derived hCG product administered intramuscularly were assessed in a double-blind, randomized, multicenter study in anovulatory infertile women (Study 8209) which was conducted in 19 centers in Australia, Canada, Europe and Israel.
The primary efficacy parameter in this single-cycle study was the patient ovulation rate. 242 patients entered the study, of whom 99 received Ovidrel® 250 µg. The efficacy of Ovidrel® 250 µg was found to be clinically and statistically equivalent to that of the approved urinary-derived hCG product. The results of those patients who received Ovidrel® 250 µg are summarized in Table 7.
| Parameter | Ovidrel® 250 µg (n = 99) |
|---|---|
|
|
| Ovulation Rate | 91 (91.9%) |
| Clinical Pregnancy Rate* | 22 (22%) |
For the 22 patients who had a clinical pregnancy with Ovidrel® 250 µg, the outcome of the pregnancy is presented in Table 8.
| Parameter | Ovidrel® 250 µg (n = 22) |
|---|---|
| Clinical Pregnancies not reaching term | 7 (31.8%) |
| Live births | 15 (68.2%) |
| Singleton | 13 (86.7%) |
| Multiple birth | 2 (13.3%) |
Indications and Usage for Ovidrel Injection
Ovidrel® PreFilled Syringe (choriogonadotropin alfa injection) is indicated for the induction of final follicular maturation and early luteinization in infertile women who have undergone pituitary desensitization and who have been appropriately pretreated with follicle stimulating hormones as part of an Assisted Reproductive Technology (ART) program such as in vitro fertilization and embryo transfer. Ovidrel® PreFilled Syringe is also indicated for the induction of ovulation (OI) and pregnancy in anovulatory infertile patients in whom the cause of infertility is functional and not due to primary ovarian failure.
Selection of Patients
- Before treatment with gonadotropins is instituted, a thorough gynecologic and endocrinologic evaluation must be performed. This should include an assessment of pelvic anatomy. Patients with tubal obstruction should receive Ovidrel® PreFilled Syringe only if enrolled in an in vitro fertilization program.
- Primary ovarian failure should be excluded by the determination of gonadotropin levels.
- Appropriate evaluation should be performed to exclude pregnancy.
- Patients in later reproductive life have a greater predisposition to endometrial carcinoma as well as a higher incidence of anovulatory disorders. A thorough diagnostic evaluation should always be performed in patients who demonstrate abnormal uterine bleeding or other signs of endometrial abnormalities before starting FSH and Ovidrel® PreFilled Syringe therapy.
- Evaluation of the partner's fertility potential should be included in the initial evaluation.
Warnings
Gonadotropins, including Ovidrel® PreFilled Syringe (choriogonado-tropin alfa injection), should only be used by physicians who are thoroughly familiar with infertility problems and their management. Like other hCG products, Ovidrel® PreFilled Syringe is a potent gonadotropic substance capable of causing Ovarian Hyperstimulation Syndrome (OHSS) in women with or without pulmonary or vascular complications. The risks of gonadoptropin treatment should be considered for women with risk factors of thromboembolic events such as prior medical or family history. Gonadotropin therapy requires a certain time commitment by physicians and supportive health professionals, and requires the availability of appropriate monitoring facilities (see "Precautions/ Laboratory Tests"). Safe and effective induction of ovulation and use of Ovidrel® PreFilled Syringe in women requires monitoring of ovarian response with serum estradiol and transvaginal ultrasound on a regular basis.
Overstimulation of the Ovary Following hCG Therapy
Precautions
Laboratory Tests
In most instances, treatment of women with FSH results only in follicular recruitment and development. In the absence of an endogenous LH surge, hCG is given when monitoring of the patient indicates that sufficient follicular development has occurred. This may be estimated by ultrasound alone or in combination with measurement of serum estradiol levels. The combination of both ultrasound and serum estradiol measurement are useful for monitoring the development of follicles, for timing of the ovulatory trigger, as well as for detecting ovarian enlargement and minimizing the risk of the Ovarian Hyperstimulation Syndrome and multiple gestation. It is recommended that the number of growing follicles be confirmed using ultrasonography because serum estrogens do not give an indication of the size or number of follicles.
Human chorionic gonadotropins can crossreact in the radioimmunoassay of gonadotropins, especially luteinizing hormone. Each individual laboratory should establish the degree of crossreactivity with their gonadotropin assay. Physicians should make the laboratory aware of patients on hCG if gonadotropin levels are requested.
The clinical confirmation of ovulation, with the exception of pregnancy, is obtained by direct and indirect indices of progesterone production. The indices most generally used are as follows:
- A rise in basal body temperature
- Increase in serum progesterone and
- Menstruation following a shift in basal body temperature
When used in conjunction with the indices of progesterone production, sonographic visualization of the ovaries will assist in determining if ovulation has occurred. Sonographic evidence of ovulation may include the following:
- Fluid in the cul-de-sac
- Ovarian stigmata
- Collapsed follicle
- Secretory endometrium
Accurate interpretation of the indices of ovulation require a physician who is experienced in the interpretation of these tests.
Adverse Reactions/Side Effects
(see WARNINGS)
The safety of Ovidrel® was examined in four clinical studies that treated 752 patients of whom 335 received Ovidrel® 250 µg following follicular recruitment with gonadotropins. When patients enrolled in four clinical studies (3 in ART and one in OI) were injected subcutaneously with either Ovidrel® or an approved urinary-derived hCG, 14.6 % (49 of 335 patients) in the Ovidrel® 250 µg group experienced application site disorders compared to 28% (92 of 328 patients) in the approved u-hCG group. Adverse events reported for Ovidrel® 250 µg occurring in at least 2% of patients (regardless of causality) are listed in Table 9 for the 3 ART studies and in Table 10 for the single OI study.
| Body System | Ovidrel® 250 µg (n=236) |
|---|---|
| Preferred Term | Incidence Rate % (n) |
| At Least One Adverse Event | 33.1% (78) |
| Application Site Disorders | 14.0% (33) |
| Injection Site Pain | 7.6% (18) |
| Injection Site Bruising | 4.7% (11) |
| Gastro-Intestinal System Disorders | 8.5% (20) |
| Abdominal Pain | 4.2% (10) |
| Nausea | 3.4% ( 8) |
| Vomiting | 2.5% ( 6) |
| Secondary Terms (Post-Operative Pain) | 4.7% (11) |
| Post-Operative Pain | 4.7% (11) |
Adverse events not listed in Table 9 that occurred in less than 2% of patients treated with Ovidrel® 250 µg whether or not considered causally related to Ovidrel®, included: injection site inflammation and reaction, flatulence, diarrhea, hiccup, ectopic pregnancy, breast pain, intermenstrual bleeding, vaginal hemorrhage, cervical lesion, leukorrhea, ovarian hyperstimulation, uterine disorders, vaginitis, vaginal discomfort, body pain, back pain, fever, dizziness, headache, hot flashes, malaise, paraesthesias, rash, emotional lability, insomnia, upper respiratory tract infection, cough, dysuria, urinary tract infection, urinary incontinence, albuminuria, cardiac arrhythmia, genital moniliasis, genital herpes, leukocytosis, heart murmur and cervical carcinoma.
| Body System | Ovidrel® 250 µg (n=99) |
|---|---|
| Preferred Term | Incidence Rate % (n) |
| At Least One Adverse Event | 26.2% (26) |
| Application Site Disorders | 16.2% (16) |
| Injection site pain | 8.1% (8) |
| Injection site inflammation | 2.0% (2) |
| Injection site bruising | 3.0% (3) |
| Injection site reaction | 3.0% (3) |
| Reproductive Disorders, Female | 7.1% (7) |
| Ovarian cyst | 3.0% (3) |
| Ovarian hyperstimulation | 3.0% (3) |
| Gastro-Intestinal System Disorders | 4.0% (4) |
| Abdominal pain | 3.0% (3) |
Additional adverse events not listed in Table 10 that occurred in less than 2% of patients treated with Ovidrel® 250 µg, whether or not considered causally related to Ovidrel®, included: breast pain, flatulence, abdominal enlargement, pharyngitis, upper respiratory tract infection, hyperglycemia and pruritis.
The following medical events have been reported subsequent to pregnancies resulting from hCG therapy in controlled clinical studies:
- Spontaneous Abortion
- Ectopic Pregnancy
- Premature Labor
- Postpartum Fever
- Congenital Abnormalities
Of 125 clinical pregnancies reported following treatment with FSH and Ovidrel® 250 µg or 500 µg, three were associated with a congenital anomaly of the fetus or newborn. Among patients receiving Ovidrel® 250 µg, cranial malformation was detected in the fetus of one woman and a chromosomal abnormality (47, XXX) in another. These events were judged by the investigators to be of unlikely or unknown relation to treatment. These three events represent an incidence of major congenital malformations of 2.4%, which is consistent with the reported rate for pregnancies resulting from natural or assisted conception. In a woman who received Ovidrel® 500 µg, one birth in a set of triplets was associated with Down's syndrome and atrial septal defect. This event was considered to be unrelated to the study drug.
The following adverse reactions have been previously reported during menotropin therapy:
- Pulmonary and vascular complications (see "Warnings")
- Adnexal torsion (as a complication of ovarian enlargement)
- Mild to moderate ovarian enlargement
- Hemoperitoneum
There have been infrequent reports of ovarian neoplasms, both benign and malignant, in women who have undergone multiple drug regimens for ovulation induction; however, a causal relationship has not been established.
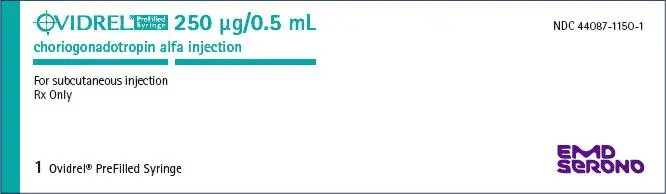
| OVIDREL
choriogonadotropin alfa injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - EMD Serono, Inc. (088514898) |