Drug Detail:Pacerone (Amiodarone (oral) [ a-mi-oh-da-rone ])
Drug Class: Group III antiarrhythmics
Highlights of Prescribing Information
PACERONE® (amiodarone hydrochloride) tablets, for oral use
Initial U.S. Approval: 1985
WARNING: PULMONARY, HEPATIC, and CARDIAC TOXICITY
See full prescribing information for complete boxed warning.
- Reserve Pacerone for patients with the indicated life-threatening arrhythmias because its use is accompanied by substantial toxicity, some also life-threatening. Utilize alternative agents first. (1)
- Pacerone' s life-threatening toxicities include pulmonary (5.2), hepatic (5.3), and proarrhythmic (5.4).
- Initiate under hospital or specialist supervision. (5)
Indications and Usage for Pacerone
Pacerone is an antiarrhythmic indicated for:
- Recurrent ventricular fibrillation. (1)
- Recurrent hemodynamically unstable ventricular tachycardia. (1)
Pacerone Dosage and Administration
Initiate treatment with a loading dose of 800 to 1600 mg/day until initial therapeutic response occurs (usually 1 to 3 weeks). Once adequate arrhythmia control is achieved, or if side effects become prominent, reduce Pacerone tablets dose to 600 to 800 mg/day for one month and then to the maintenance dose, usually 400 mg/day. (2)
Dosage Forms and Strengths
Tablets: 100 mg, 200 mg and 400 mg (3)
Contraindications
Pacerone is contraindicated in patients with (4):
- Cardiogenic shock.
- Sick sinus syndrome, second- or third-degree AV block, bradycardia leading to syncope without a functioning pacemaker.
- Known hypersensitivity to the drug or any of its components.
Warnings and Precautions
- Persistence of Adverse Effects: Adverse reactions and drug interaction can persist for several weeks following discontinuation. (5.1)
- Impaired Vision: Corneal microdeposits (common; reversible), optic neuropathy/neuritis (rare; may lead to blindness). (5.5)
- Thyroid Abnormalities: Hyperthyroidism or hypothyroidism. (5.6)
Adverse Reactions/Side Effects
- The most common reactions (>1%) leading to discontinuation of amiodarone include pulmonary toxicity, paroxysmal ventricular tachycardia, congestive heart failure, and elevation of liver enzymes. (6)
To report SUSPECTED ADVERSE REACTIONS, contact Upsher-Smith Laboratories, LLC at 1-855-899-9180 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Avoid coadministration of amiodarone with other antiarrhythmics and drugs known to prolong the QT interval. (7)
- Amiodarone is a substrate for CYP3A and CYP2C8, so inhibitors and inducers affect amiodarone exposure. (7)
- Amiodarone inhibits P-glycoprotein and CYP1A2, CYP2C9, CYP2D6, and CYP3A, increasing exposure to other drugs. (7)
Use In Specific Populations
- Pregnancy: May cause fetal harm. (8.1)
- Lactation: Breastfeeding not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2023
Related/similar drugs
diltiazem, amiodarone, lidocaine, bisoprolol, verapamil, flecainide, phenylephrineFull Prescribing Information
WARNING: PULMONARY, HEPATIC and CARDIAC TOXICITY
Pacerone is intended for use only in patients with the indicated life-threatening arrhythmias because its use is accompanied by substantial toxicity [see Indications and Usage (1)].
Pacerone can cause pulmonary toxicity (hypersensitivity pneumonitis or interstitial/alveolar pneumonitis) that has resulted in clinically manifest disease at rates as high as 17% in some series of patients. Pulmonary toxicity has been fatal about 10% of the time. Obtain a baseline chest X-ray and pulmonary-function tests, including diffusion capacity, when Pacerone therapy is initiated. Repeat history, physical exam, and chest X-ray every 3 to 6 months [see Warnings and Precautions 5.2)].
Pacerone can cause hepatoxicity, which can be fatal. Obtain baseline and periodic liver transaminases and discontinue or reduce dose if the increase exceeds three times normal, or doubles in a patient with an elevated baseline. Discontinue Pacerone if the patient experiences signs or symptoms of clinical liver injury [see Warnings and Precautions (5.3)].
Pacerone can exacerbate arrhythmias. Initiate amiodarone hydrochloride in a clinical setting where continuous electrocardiograms and cardiac resuscitation are available [see Warnings and Precautions (5.4)].
1. Indications and Usage for Pacerone
Pacerone is indicated for the treatment of documented, life-threatening recurrent ventricular fibrillation and life-threatening recurrent hemodynamically unstable tachycardia in adults who have not responded to adequate doses of other available antiarrhythmics or when alternative agents cannot be tolerated.
2. Pacerone Dosage and Administration
Dosage must be individualized based on severity of arrhythmia and response. Use the lowest effective dose. Obtain baseline chest x-ray, pulmonary function tests, thyroid function tests, and liver aminotransferases. Correct hypokalemia, hypomagnesemia, and hypocalcemia before initiating treatment.
3. Dosage Forms and Strengths
Pacerone tablets, 100 mg, are peach, round, flat-faced, uncoated tablets, debossed with "P" on one side, and "U-S" above "144" on the other side.
Pacerone tablets, 200 mg, are pink, round, flat-faced, scored, uncoated tablets, debossed with "P200" on the unscored side, and "U-S" above and "0147" below the score on the reverse side.
Pacerone tablets, 400 mg, are white to off-white, round, uncoated, scored tablets, debossed with "U-S' above and "1645' below the score on one side and "P400" on the unscored side.
4. Contraindications
- Cardiogenic shock.
- Sick sinus syndrome, second- or third-degree atrioventricular block, bradycardia leading to syncope without a functioning pacemaker.
- Known hypersensitivity to the drug or to any of its components, including iodine.
5. Warnings and Precautions
5.1 Persistence of Adverse Effects
Because of the long half-life of amiodarone (15 to 142 days) and its active metabolite desethylamiodarone (14 to 75 days), adverse reactions and drug interactions can persist for several weeks following amiodarone discontinuation [see Clinical Pharmacology (12.3)].
5.2 Pulmonary Toxicity
Pacerone may cause a clinical syndrome of cough and progressive dyspnea accompanied by functional, radiographic, gallium-scan, and pathological data consistent with pulmonary toxicity. Pulmonary toxicity secondary to Pacerone may result from either indirect or direct toxicity as represented by hypersensitivity pneumonitis (including eosinophilic pneumonia) or interstitial/alveolar pneumonitis, respectively. Rates of pulmonary toxicity have been reported to be as high as 17% and is fatal in about 10% of cases. Obtain a baseline chest X-ray and pulmonary-function tests, including diffusion capacity, when Pacerone therapy is initiated. Repeat history, physical exam, and chest X-ray every 3 to 6 months or if symptoms occur. Consider alternative antiarrhythmic therapy if the patient experiences signs or symptoms of pulmonary toxicity. Prednisone 40 to 60 mg/day tapered over several weeks may be helpful in treating pulmonary toxicity.
5.3 Hepatic Injury
Asymptomatic elevations of hepatic enzyme levels are seen frequently, but Pacerone can cause life-threatening hepatic injury. Histology has resembled that of alcoholic hepatitis or cirrhosis. Obtain baseline and periodic liver transaminases. If transaminases exceed three times normal, or doubles in a patient with an elevated baseline, discontinue or reduce dose of Pacerone, obtain follow-up tests and treat appropriately.
5.4 Worsened Arrhythmia
Pacerone can exacerbate the presenting arrhythmia in about 2% to 5% of patients or cause new ventricular fibrillation, incessant ventricular tachycardia, increased resistance to cardioversion, and polymorphic ventricular tachycardia associated with QTc prolongation (Torsade de Pointes [TdP]).
Correct hypokalemia, hypomagnesemia, and hypocalcemia before initiating treatment with amiodarone hydrochloride, as these disorders can exaggerate the degree of QTc prolongation and increase the potential for TdP. Give special attention to electrolyte and acid-base balance in patients experiencing severe or prolonged diarrhea or receiving drugs affecting electrolyte levels, such as diuretics, laxatives, systemic corticosteroids, or amphotericin B.
5.6 Thyroid Abnormalities
Amiodarone hydrochloride inhibits peripheral conversion of thyroxine (T4) to triiodothyronine (T3) and may cause increased thyroxine levels, decreased T3 levels, and increased levels of inactive reverse T3 (rT3) in clinically euthyroid patients. Amiodarone hydrochloride can cause either hypothyroidism (reported in up to 10% of patients) or hyperthyroidism (occurring in about 2% of patients). Monitor thyroid function prior to treatment and periodically thereafter, particularly in elderly patients, and in any patient with a history of thyroid nodules, goiter, or other thyroid dysfunction.
Hyperthyroidism may induce arrhythmia breakthrough. If any new signs of arrhythmia appear, the possibility of hyperthyroidism should be considered. Antithyroid drugs, β-adrenergic blockers, temporary corticosteroid therapy may be necessary to treat the symptoms of hyperthyroidism. The action of antithyroid drugs may be delayed in amiodarone-induced thyrotoxicosis because of substantial quantities of preformed thyroid hormones stored in the gland. Radioactive iodine therapy is contraindicated because of the low radioiodine uptake associated with amiodarone-induced hyperthyroidism. Pacerone-induced hyperthyroidism may be followed by a transient period of hypothyroidism.
Hypothyroidism may be primary or subsequent to resolution of preceding amiodarone-induced hyperthyroidism. Severe hypothyroidism and myxedema coma, sometimes fatal, have been reported in association with amiodarone therapy. In some clinically hypothyroid amiodarone-treated patients, free thyroxine index values may be normal. Manage hypothyroidism by reducing the dose of or discontinuing Pacerone and thyroid hormone supplementation.
5.7 Bradycardia
Pacerone causes symptomatic bradycardia or sinus arrest with suppression of escape foci in 2% to 4% of patients. The risk is increased by electrolytic disorders or use of concomitant antiarrhythmics or negative chronotropes [see Drug Interactions (7)]. Bradycardia may require a pacemaker for rate control.
Post-marketing cases of symptomatic bradycardia, some requiring pacemaker insertion and at least one fatal, have been reported when ledipasvir/sofosbuvir or sofosbuvir with simeprevir were initiated in patients on amiodarone. Bradycardia generally occurred within hours to days, but in some cases presented up to 2 weeks after initiating antiviral treatment. Bradycardia generally resolved after discontinuation of antiviral treatment. The mechanism for this effect is unknown. Monitor heart rate in patients taking or recently discontinuing amiodarone when starting antiviral treatment [see Drug Interactions (7)].
5.8 Implantable Cardiac Devices
In patients with implanted defibrillators or pacemakers, chronic administration of antiarrhythmic drugs may affect pacing or defibrillation thresholds. Therefore, at the inception of and during amiodarone treatment, pacing and defibrillation thresholds should be assessed.
5.9 Fetal Toxicity
Pacerone may cause fetal harm when administered to a pregnant woman. Fetal exposure may increase the potential for cardiac, thyroid, neurodevelopmental, neurological, and growth effects in neonate [see Use in Specific Populations (8.1)].
5.10 Peripheral Neuropathy
Chronic administration of Pacerone may lead to peripheral neuropathy, which may not resolve when Pacerone is discontinued.
5.11 Photosensitivity and Skin Discoloration
Pacerone induces photosensitization in about 10% of patients; some protection may be afforded sun-barrier creams or protective clothing. During long-term treatment, a blue-gray discoloration of the exposed skin may occur. The risk may be increased in patients of fair complexion or those with excessive sun exposure. Some reversal of discoloration may occur upon drug discontinuation.
6. Adverse Reactions/Side Effects
The following serious adverse reactions are described in more detail in other sections of the prescribing information:
- Pulmonary Toxicity [see Warnings and Precautions (5.2)]
- Hepatic Injury [see Warnings and Precautions (5.3)]
- Worsened Arrhythmia [see Warnings and Precautions (5.4)]
- Visual Impairment and Loss of Vision [see Warnings and Precautions (5.5)]
- Thyroid Abnormalities [see Warnings and Precautions (5.6)]
- Bradycardia [see Warnings and Precautions (5.7)]
- Peripheral Neuropathy [see Warnings and Precautions (5.10)]
- Photosensitivity and Skin Discoloration [see Warnings and Precautions (5.11)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
At the usual maintenance dose (400 mg/day) and above, amiodarone hydrochloride causes adverse reactions in about three-fourths of all patients, resulting in discontinuation in 7% to 18%.
In surveys of almost 5,000 patients treated in open U.S. studies and in published reports of treatment with amiodarone hydrochloride, the adverse reactions most frequently requiring discontinuation of amiodarone hydrochloride included pulmonary infiltrates or fibrosis, paroxysmal ventricular tachycardia, congestive heart failure, and elevation of liver enzymes. Other symptoms causing discontinuations less often included visual disturbances, photosensitivity, blue skin discoloration, hyperthyroidism, and hypothyroidism.
The following side-effect rates are based on a retrospective study of 241 patients treated for 2 to 1,515 days (mean 441.3 days):
Thyroid
Common: Hypothyroidism, hyperthyroidism.
Cardiovascular
Common: Congestive heart failure, cardiac arrhythmias, SA node dysfunction.
Gastrointestinal
Very common: Nausea, vomiting.
Common: Constipation, anorexia, abdominal pain.
Dermatologic
Common: Solar dermatitis/photosensitivity.
Neurologic
Common: Malaise and fatigue, tremor/abnormal involuntary movements, lack of coordination, abnormal gait/ataxia, dizziness, paresthesias, decreased libido, insomnia, headache, sleep disturbances.
Ophthalmic
Common: Visual disturbances.
Hepatic
Common: Abnormal liver-function tests, nonspecific hepatic disorders.
Respiratory
Common: Pulmonary inflammation or fibrosis.
Other
Common: Flushing, abnormal taste and smell, edema, abnormal salivation, coagulation abnormalities.
Uncommon: Blue skin discoloration, rash, spontaneous ecchymosis, alopecia, hypotension, and cardiac conduction abnormalities.
7. Drug Interactions
Because of amiodarone's long half-life, expect drug interactions to persist for weeks to months after discontinuation of amiodarone.
Drug interactions with amiodarone are described in Table 1 below.
| Concomitant Drug Class/Name | Examples | Clinical Comment |
|---|---|---|
| Pharmacodynamic Interactions | ||
| QT Prolonging Drugs | class I and III antiarrhythmics, lithium, certain phenothiazines, tricyclic antidepressants, certain fluoroquinolone and macrolide antibiotics, azole antifungals, halogenated inhalation anesthetic agents | Increased risk of Torsade de Pointes. Avoid concomitant use. |
| Negative Chronotropes | digoxin, beta blockers, verapamil, diltiazem, clonidine, ivabradine | Potentiates the electrophysiologic and hemodynamic effects of amiodarone, resulting in bradycardia, sinus arrest, and AV block. Monitor heart rate. |
| Pharmacokinetic Interactions | ||
| CYP450 Inhibitors | grapefruit juice, certain fluoroquinolone and macrolide antibiotics, azole antifungals, cimetidine, certain protease inhibitors | Increased exposure of amiodarone. Avoid concomitant use. |
| CYP450 Inducers | St. John's Wort | Reduced amiodarone serum levels. |
| Cyclosporine | Increased plasma levels of cyclosporine have been reported resulting in elevated creatinine, despite reduction of cyclosporine dose. Monitor cyclosporine drug levels and renal function with concomitant use. | |
| Cholestyramine | Reduced amiodarone serum levels. | |
| Antiarrhythmics | quinidine, procainamide, flecainide | Reserve concomitant use for patients who are unresponsive to a single agent. Antiarrhythmic metabolism inhibited by amiodarone. Initiate antiarrhythmic at a lower than usual dose and monitor patient carefully. Reduce dose levels of previously administered antiarrhythmic by 30% to 50% for several days after transitioning to oral amiodarone. Evaluate continued need for antiarrhythmic. |
| Digoxin | Increased digoxin concentration. Reduce digoxin by half or discontinue. If continued, monitor for evidence of toxicity. | |
| HMG-CoA Reductase Inhibitors | simvastatin, lovastatin, atorvastatin | Increased plasma concentration of HMG-CoA reductase inhibitor. Limit the dose of lovastatin to 40 mg. Limit the coadministered dose of simvastatin to 20 mg. Lower starting dose of other CYP3A4 substrates may be required. |
| Warfarin | Potentiates anticoagulant response and can result in serious or fatal bleeding. Coadministration increases prothrombin time by 100% after 3 to 4 days. Reduce warfarin dose by one-third to one-half and monitor prothrombin times. | |
| Phenytoin | Increased steady-state levels of phenytoin. Monitor phenytoin levels. | |
| Hepatitis C Direct Acting Antiviral | sofosbuvir | Cases of symptomatic bradyarrhythmia requiring pacemaker insertion have been reported in patients on oral maintenance amiodarone who initiated therapy with sofosbuvir. |
| CYP3A Substrate | lidocaine | Sinus bradycardia has been reported with oral amiodarone in combination with lidocaine given for local anesthesia. Monitor heart rate. A lower starting dose of lidocaine may be required. |
| CYP3A Substrate | fentanyl | Fentanyl in combination with amiodarone may cause hypotension, bradycardia, and decreased cardiac output. |
8. Use In Specific Populations
8.4 Pediatric Use
The safety and effectiveness of Pacerone in pediatric patients have not been established.
8.5 Geriatric Use
Normal subjects over 65 years of age show lower clearances and increased drug half-life than younger subjects [see Clinical Pharmacology (12.3)]. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
10. Overdosage
There have been cases, some fatal, of amiodarone hydrochloride overdose.
Monitor the patient's cardiac rhythm and blood pressure, and, if bradycardia ensues, a β-adrenergic agonist or a pacemaker may be used. Treat hypotension with inadequate tissue perfusion with positive inotropic and vasopressor agents. Neither Pacerone nor its metabolite is dialyzable.
11. Pacerone Description
Pacerone (amiodarone hydrochloride tablets, USP) are an antiarrhythmic drug, available for oral administration as 100 mg; peach tablets, 200 mg; pink tablets and 400 mg; white tablets containing amiodarone hydrochloride, USP. All three strengths of Pacerone tablets contain the following inactive ingredients: lactose monohydrate, magnesium stearate, povidone, pregelatinized corn starch, sodium starch glycolate and stearic acid. The 200 mg tablets also contain FD&C Red No. 40 and FD&C Yellow No. 6. The 100 mg tablets also contain FD&C Yellow No. 6.
Amiodarone hydrochloride, USP is a benzofuran derivative: 2-butyl-3-benzofuranyl 4-[2-(diethylamino)-ethoxy]-3,5-diiodophenyl ketone hydrochloride.
The structural formula is as follows:
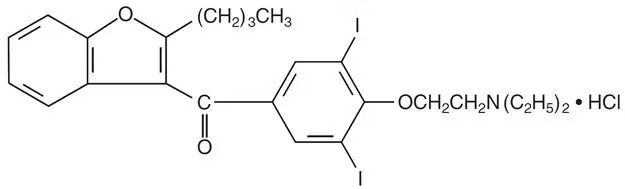
C25H29I2NO3 ∙ HCl Molecular Weight: 681.8
C25H29I2NO3 ∙ HCl Molecular Weight: 681.8
Amiodarone hydrochloride, USP is a white to cream-colored crystalline powder. It is slightly soluble in water, soluble in alcohol, and freely soluble in chloroform. It contains 37.3% iodine by weight.
Meets USP Dissolution Test 4.
12. Pacerone - Clinical Pharmacology
12.1 Mechanism of Action
Amiodarone is considered a class III antiarrhythmic drug, but it possesses electrophysiologic characteristics of all four Vaughan Williams classes. Like class I drugs, amiodarone blocks sodium channels at rapid pacing frequencies, and like class II drugs, amiodarone exerts a noncompetitive antisympathetic action. One of its main effects, with prolonged administration, is to lengthen the cardiac action potential, a class III effect. The negative chronotropic effect of amiodarone in nodal tissues is similar to the effect of class IV drugs. In addition to blocking sodium channels, amiodarone blocks myocardial potassium channels, which contributes to slowing of conduction and prolongation of refractoriness. The antisympathetic action and the block of calcium and potassium channels are responsible for the negative dromotropic effects on the sinus node and for the slowing of conduction and prolongation of refractoriness in the atrioventricular (AV) node. Its vasodilatory action can decrease cardiac workload and consequently myocardial oxygen consumption.
Pacerone prolongs the duration of the action potential of all cardiac fibers while causing minimal reduction of dV/dt (maximal upstroke velocity of the action potential). The refractory period is prolonged in all cardiac tissues. Amiodarone hydrochloride increases the cardiac refractory period without influencing resting membrane potential, except in automatic cells where the slope of the prepotential is reduced, generally reducing automaticity. These electrophysiologic effects are reflected in a decreased sinus rate of 15% to 20%, increased PR and QT intervals of about 10%, the development of U-waves, and changes in T-wave contour. These changes should not require discontinuation of Pacerone as they are evidence of its pharmacological action, although Pacerone can cause marked sinus bradycardia or sinus arrest and heart block [see Warnings and Precautions (5.4)].
12.2 Pharmacodynamics
There is no well-established relationship between plasma concentration and effectiveness, but it does appear that concentrations much below 1 mg/L are often ineffective and that levels above 2.5 mg/L are generally not needed. Plasma-concentration measurements can be used to identify patients whose levels are unusually low, and who might benefit from a dose increase, or unusually high, and who might have dosage reduction in the hope of minimizing side effects.
Effects on abnormal rhythms are not seen before 2 to 3 days and usually require 1 to 3 weeks, even when a loading dose is used. There may be a continued increase in effect for longer periods still. There is evidence that the time to effect is shorter when a loading-dose regimen is used.
Consistent with the slow rate of elimination, antiarrhythmic effects persist for weeks or months after amiodarone hydrochloride is discontinued, but the time of recurrence is variable and unpredictable. In general, when the drug is resumed after recurrence of the arrhythmia, control is established relatively rapidly compared to the initial response, presumably because tissue stores were not wholly depleted.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Amiodarone hydrochloride was associated with a statistically significant, dose-related increase in the incidence of thyroid tumors (follicular adenoma and/or carcinoma) in rats. The incidence of thyroid tumors was greater than control at the lowest dose level tested, i.e., 5 mg/kg/day (approximately 0.08 times the maximum recommended human maintenance dose2).
Mutagenicity studies (Ames, micronucleus, and lysogenic tests) with amiodarone hydrochloride were negative.
In a study in which amiodarone hydrochloride was administered to male and female rats, beginning 9 weeks prior to mating, reduced fertility was observed at a dose level of 90 mg/kg/day (approximately 1.4 times the maximum recommended human maintenance dose2).
- 2
- 600 mg in a 60 kg patient (dose compared on a body surface area basis)
16. How is Pacerone supplied
Pacerone® (amiodarone hydrochloride tablets, USP) 100 mg are peach, round, flat-faced, uncoated tablets, debossed with "P" on one side, and "U-S" above "144" on the other side. They are supplied as follows:
Bottles of 30 with child-resistant closure, NDC 0245-0144-30
Bottles of 100 with child-resistant closure, NDC 0245-0144-11
Unit-dose cartons of 100 tablets, NDC 0245-0144-01
Pacerone® (amiodarone hydrochloride tablets, USP) 200 mg, are pink, round, flat-faced, scored, uncoated tablets, debossed with "P200" on the unscored side, and "U-S" above and "0147" below the score on the reverse side. They are supplied as follows:
Bottles of 60 with child-resistant closure, NDC 0245-0147-60
Bottles of 90 with child-resistant closure, NDC 0245-0147-90
Bottles of 500 tablets, NDC 0245-0147-15
Unit-dose cartons of 100 tablets, NDC 0245-0147-01
Pacerone® (amiodarone hydrochloride tablets, USP) 400 mg, are white to off-white, round, uncoated, scored tablets, debossed with "U-S" above and "1645" below the score on one side and "P400" on the unscored side. They are supplied as follows:
Bottles of 30 with child-resistant closure, NDC 0245-1645-30
Unit-dose cartons of 100 tablets, NDC 0245-1645-01
Keep tightly closed.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to inform their prescriber of a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Advise women that breastfeeding is not recommended during treatment with Pacerone [see Use in Specific Populations (8.2)].
Advise patients to avoid grapefruit juice and St. John's Wort.
Advise patients to seek medical attention if they experience the signs and symptoms of pulmonary toxicity, worsening arrhythmia, bradycardia, visual impairment, or hypo- and hyperthyroidism.
This product's label may have been updated. For full prescribing information, please visit www.upsher-smith.com.
| MEDICATION GUIDE Pacerone® (PĀS-ər-ōn) Tablets (Amiodarone Hydrochloride) |
||
|---|---|---|
| What is the most important information I should know about Pacerone tablets?
Pacerone tablets can cause serious side effects that can lead to death, including:
Pacerone tablets should only be used to treat people who have been diagnosed with life-threatening heartbeat problems called ventricular arrhythmias, when other treatments did not work or you cannot tolerate them. Pacerone tablets can cause other serious side effects. See "What are the possible side effects of Pacerone tablets?" If you get serious side effects during treatment you may need to stop Pacerone tablets, have your dose changed, or get medical treatment. Talk with your healthcare provider before you stop taking Pacerone tablets. You may still have side effects after stopping Pacerone tablets because the medicine stays in your body for months after treatment is stopped. You should have regular check-ups, blood tests, chest x-rays before and during treatment with Pacerone tablets to check for serious side effects. You should also have lung function tests before starting treatment with Pacerone tablets. |
||
| What are Pacerone tablets?
Pacerone tablets are a prescription medicine used to treat people who have been diagnosed with life-threatening heartbeat problems called ventricular arrhythmias, when other treatments did not work or you cannot tolerate them. It is not known if Pacerone tablets are safe and effective in children. |
||
| Who should not take Pacerone tablets? Do not take Pacerone tablets if you:
|
||
Before taking Pacerone tablets, tell your healthcare provider about all of your medical conditions, including if you:
|
||
How should I take Pacerone tablets?
|
||
What should I avoid while taking Pacerone tablets?
|
||
| What are the possible side effects of Pacerone tablets? Pacerone tablets can cause serious side effects, including:
|
||
|
|
|
The most common side effects of Pacerone tablets include:
|
||
| Pacerone tablets may affect fertility in males and females. It is not known if the effects are reversible. Talk to your healthcare provider if you have concerns about fertility. These are not all the possible side effects of Pacerone tablets. For more information, ask your healthcare provider or pharmacist. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
How should I store Pacerone tablets?
|
||
| General information about the safe and effective use of Pacerone tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use Pacerone tablets for a condition for which it was not prescribed. Do not give Pacerone tablets to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about Pacerone tablets that is written for health professionals. |
||
| What are the ingredients in Pacerone tablets?
Active Ingredient: amiodarone hydrochloride Inactive Ingredients: lactose monohydrate, magnesium stearate, povidone, pregelatinized corn starch, sodium starch glycolate, stearic acid, FD&C Red No. 40 (200 mg) and FD&C Yellow No. 6 (100 mg and 200 mg). For more information and the most current Medication Guide, please visit www.upsher-smith.com or call 1-888-650-3789. |
||
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Manufactured by
UPSHER-SMITH LABORATORIES, LLC
Maple Grove, MN 55369
Pacerone is a registered trademark of Upsher-Smith Laboratories, LLC.
Revised 5/2020
| PACERONE
amiodarone hydrochloride tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| PACERONE
amiodarone hydrochloride tablet |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PACERONE
amiodarone hydrochloride tablet |
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
|
|||||||||||||||||||||||||
| Labeler - Upsher-Smith Laboratories, LLC (079111820) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Upsher-Smith Laboratories, LLC | 079111820 | MANUFACTURE(0245-0144, 0245-0147, 0245-1645) , PACK(0245-0144, 0245-0147, 0245-1645) , LABEL(0245-0144, 0245-0147, 0245-1645) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Upsher-Smith Laboratories, LLC | 047251004 | ANALYSIS(0245-0144, 0245-0147, 0245-1645) | |







