Drug Detail:Pifeltro (Doravirine [ dor-a-vir-een ])
Drug Class: NNRTIs
Highlights of Prescribing Information
PIFELTRO™ (doravirine) tablets, for oral use
Initial U.S. Approval: 2018
Recent Major Changes
| Indications and Usage (1) | 01/2022 |
| Dosage and Administration (2), Recommended Dosage (2.1) | 01/2022 |
Indications and Usage for Pifeltro
PIFELTRO, a non-nucleoside reverse transcriptase inhibitor (NNRTI), is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 35 kg:
- with no prior antiretroviral treatment history, OR
- to replace the current antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen with no history of treatment failure and no known substitutions associated with resistance to doravirine. (1)
Pifeltro Dosage and Administration
- Recommended dosage: One tablet taken orally once daily with or without food in adults and pediatric patients weighing at least 35 kg. (2.1)
- Dosage adjustment with rifabutin: One tablet taken twice daily (approximately 12 hours apart). (2.2)
Dosage Forms and Strengths
- Tablets: 100 mg doravirine. (3)
Contraindications
- PIFELTRO is contraindicated when co-administered with drugs that are strong cytochrome P450 (CYP)3A enzyme inducers as significant decreases in doravirine plasma concentrations may occur, which may decrease the effectiveness of PIFELTRO. (4)
Warnings and Precautions
- Monitor for Immune Reconstitution Syndrome. (5.2)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence greater than or equal to 5%, all grades) are nausea, dizziness, headache, fatigue, diarrhea, abdominal pain, and abnormal dreams. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme LLC at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Drug Interactions
Consult the full prescribing information prior to and during treatment for important potential drug-drug interactions. (4, 5.1, 7)
Use In Specific Populations
- Lactation: Breastfeeding is not recommended due to the potential for HIV-1 transmission. (8.2)
- Pediatrics: Not recommended for patients weighing less than 35 kg. (8.4)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 6/2022
Related/similar drugs
Biktarvy, Descovy, Truvada, tenofovir, Atripla, Complera, StribildFull Prescribing Information
1. Indications and Usage for Pifeltro
PIFELTRO™ is indicated in combination with other antiretroviral agents for the treatment of HIV-1 infection in adults and pediatric patients weighing at least 35 kg:
- with no prior antiretroviral treatment history; OR
- to replace the current antiretroviral regimen in those who are virologically-suppressed (HIV-1 RNA less than 50 copies per mL) on a stable antiretroviral regimen with no history of treatment failure and no known substitutions associated with resistance to doravirine [see Clinical Studies (14)].
2. Pifeltro Dosage and Administration
3. Dosage Forms and Strengths
PIFELTRO film-coated tablets are white, oval-shaped tablets, debossed with the corporate logo and 700 on one side and plain on the other side. Each tablet contains 100 mg doravirine.
4. Contraindications
PIFELTRO is contraindicated when co-administered with drugs that are strong cytochrome P450 (CYP)3A enzyme inducers as significant decreases in doravirine plasma concentrations may occur, which may decrease the effectiveness of PIFELTRO [see Warnings and Precautions (5.1), Drug Interactions (7.1), and Clinical Pharmacology (12.3)]. These drugs include, but are not limited to, the following:
- the anticonvulsants carbamazepine, oxcarbazepine, phenobarbital, phenytoin
- the androgen receptor inhibitor enzalutamide
- the antimycobacterials rifampin, rifapentine
- the cytotoxic agent mitotane
- St. John's wort (Hypericum perforatum)
5. Warnings and Precautions
5.1 Risk of Adverse Reactions or Loss of Virologic Response Due to Drug Interactions
The concomitant use of PIFELTRO and certain other drugs may result in known or potentially significant drug interactions, some of which may lead to loss of therapeutic effect of PIFELTRO and possible development of resistance [see Dosage and Administration (2.2), Contraindications (4) and Drug Interactions (7.1)].
See Table 6 for steps to prevent or manage these possible and known significant drug interactions, including dosing recommendations. Consider the potential for drug interactions prior to and during PIFELTRO therapy, review concomitant medications during PIFELTRO therapy, and monitor for adverse reactions.
5.2 Immune Reconstitution Syndrome
Immune reconstitution syndrome has been reported in patients treated with combination antiretroviral therapy. During the initial phase of combination antiretroviral treatment, patients whose immune system responds may develop an inflammatory response to indolent or residual opportunistic infections (such as Mycobacterium avium infection, cytomegalovirus, Pneumocystis jirovecii pneumonia (PCP), or tuberculosis), which may necessitate further evaluation and treatment.
Autoimmune disorders (such as Graves' disease, polymyositis, Guillain-Barré syndrome, and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution; however, the time to onset is more variable and can occur many months after initiation of treatment.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in other sections of the labeling:
- Immune Reconstitution Syndrome [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
Adverse Reactions in Adults with No Antiretroviral Treatment History
The safety assessment of PIFELTRO used in combination with other antiretroviral agents is based on Week 96 data from two Phase 3, randomized, international, multicenter, double-blind, active-controlled trials (DRIVE-FORWARD (Protocol 018) and DRIVE-AHEAD (Protocol 021)).
In DRIVE-FORWARD, 766 adult subjects received either PIFELTRO 100 mg (n=383) or darunavir 800 mg + ritonavir 100 mg (DRV+r) (n=383) once daily, each in combination with emtricitabine/tenofovir disoproxil fumarate (FTC/TDF) or abacavir/lamivudine (ABC/3TC). By Week 96, 2% in the PIFELTRO group and 3% in the DRV+r group had adverse events leading to discontinuation of study medication.
In DRIVE-AHEAD, 728 adult subjects received either DELSTRIGO [doravirine (DOR)/3TC/TDF] (n=364) or efavirenz (EFV)/FTC/TDF once daily (n=364). By Week 96, 3% in the DELSTRIGO group and 7% in the EFV/FTC/TDF group had adverse events leading to discontinuation of study medication.
Adverse reactions reported in greater than or equal to 5% of subjects in any treatment group in DRIVE-FORWARD and DRIVE-AHEAD are presented in Table 1.
| DRIVE-FORWARD | DRIVE-AHEAD | |||
|---|---|---|---|---|
| PIFELTRO+2 NRTIs‡
Once Daily N=383 | DRV+r+2 NRTIs‡
Once Daily N=383 | DELSTRIGO Once Daily N=364 | EFV/FTC/TDF Once Daily N=364 |
|
| NRTIs = FTC/TDF or ABC/3TC. Fatigue: includes fatigue, asthenia, malaise Abdominal Pain: includes abdominal discomfort, abdominal pain, abdominal pain lower, abdominal pain upper, epigastric discomfort Rash: includes rash, rash erythematous, rash generalized, rash macular, rash maculo-papular, rash papular, rash pruritic, rash pustular |
||||
|
||||
| Nausea | 7% | 8% | 5% | 7% |
| Headache | 6% | 3% | 4% | 5% |
| Fatigue | 6% | 3% | 4% | 4% |
| Diarrhea | 6% | 13% | 4% | 6% |
| Abdominal Pain | 5% | 2% | 1% | 2% |
| Dizziness | 3% | 2% | 7% | 32% |
| Rash | 2% | 3% | 2% | 12% |
| Abnormal Dreams | 1% | <1% | 5% | 10% |
| Insomnia | 1% | 2% | 4% | 5% |
| Somnolence | 0% | <1% | 3% | 7% |
The majority (77%) of adverse reactions associated with doravirine occurred at severity Grade 1 (mild).
Change in Lipids from Baseline
For DRIVE-FORWARD and DRIVE-AHEAD, changes from baseline at Week 48 in LDL-cholesterol, non-HDL-cholesterol, total cholesterol, triglycerides, and HDL-cholesterol are shown in Table 4. Changes from baseline at Week 96 were similar to those seen at Week 48.
The LDL and non-HDL comparisons were pre-specified and are summarized in Table 4. The differences were statistically significant, showing superiority for doravirine for both parameters. The clinical benefit of these findings has not been demonstrated.
| Subjects on lipid-lowering agents at baseline were excluded from these analyses (in DRIVE-FORWARD: PIFELTRO n=12 and DRV+r n=14; in DRIVE-AHEAD: DELSTRIGO n=15 and EFV/FTC/TDF n=10). Subjects initiating a lipid-lowering agent post-baseline had their last fasted on-treatment value (prior to starting the agent) carried forward (in DRIVE-FORWARD: PIFELTRO n=6 and DRV+r n=4; in DRIVE-AHEAD: DELSTRIGO n=3 and EFV/FTC/TDF n=8). | |||||
|
|||||
| DRIVE-FORWARD | |||||
| PIFELTRO+2 NRTIs Once Daily N=320 | DRV+r+2 NRTIs Once Daily N=311 | ||||
| Laboratory Parameter Preferred Term | Baseline | Change | Baseline | Change | Difference Estimates (95% CI) |
| LDL-Cholesterol (mg/dL)* | 91.4 | -4.6 | 92.3 | 9.5 | -14.4 (-18.0, -10.8) |
| Non-HDL Cholesterol (mg/dL)* | 113.6 | -5.4 | 114.5 | 13.7 | -19.4 (-23.4, -15.4) |
| Total Cholesterol (mg/dL)† | 157.2 | -1.4 | 157.8 | 18.0 | - |
| Triglycerides (mg/dL)† | 111.0 | -3.1 | 113.7 | 24.5 | - |
| HDL-Cholesterol (mg/dL)† | 43.6 | 4.0 | 43.3 | 4.3 | - |
| DRIVE-AHEAD | |||||
| DELSTRIGO Once Daily N=320 | EFV/FTC/TDF Once Daily N=307 | ||||
| Laboratory Parameter Preferred Term | Baseline | Change | Baseline | Change | Difference Estimates (95% CI) |
| LDL-Cholesterol (mg/dL)* | 91.7 | -2.1 | 91.3 | 8.3 | -10.2 (-13.8, -6.7) |
| Non-HDL Cholesterol (mg/dL)* | 114.7 | -4.1 | 115.3 | 12.7 | -16.9 (-20.8, -13.0) |
| Total Cholesterol (mg/dL)† | 156.8 | -2.2 | 156.8 | 21.1 | - |
| Triglycerides (mg/dL)† | 118.7 | -12.0 | 122.6 | 21.6 | - |
| HDL-Cholesterol (mg/dL)† | 42.1 | 1.8 | 41.6 | 8.4 | - |
Adverse Reactions in Virologically-Suppressed Adults
The safety of DELSTRIGO in virologically-suppressed adults was based on Week 48 data from 670 subjects in the DRIVE-SHIFT trial (Protocol 024), a randomized, international, multicenter, open-label trial in which virologically-suppressed subjects were switched from a baseline regimen consisting of two NRTIs in combination with a protease inhibitor (PI) plus either ritonavir or cobicistat, or elvitegravir plus cobicistat, or a non-nucleoside reverse transcriptase inhibitor (NNRTI) to DELSTRIGO. Overall, the safety profile in virologically-suppressed adult subjects was similar to that in subjects with no antiretroviral treatment history.
7. Drug Interactions
7.1 Effect of Other Drugs on PIFELTRO
Co-administration of PIFELTRO with a CYP3A inducer decreases doravirine plasma concentrations, which may reduce PIFELTRO efficacy [see Contraindications (4), Warnings and Precautions (5.1), and Clinical Pharmacology (12.3)]. Co-administration of PIFELTRO and drugs that are inhibitors of CYP3A may result in increased plasma concentrations of doravirine.
Table 6 shows significant drug interactions with PIFELTRO.
| Concomitant Drug Class: Drug Name | Effect on Concentration | Clinical Comment |
|---|---|---|
| ↑ = increase, ↓ = decrease All other drug-drug interactions shown are anticipated based on the known metabolic and elimination pathways. |
||
|
||
| Androgen Receptors | ||
| enzalutamide | ↓ doravirine | Co-administration is contraindicated with enzalutamide. At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
| Anticonvulsants | ||
| carbamazepine oxcarbazepine phenobarbital phenytoin | ↓ doravirine | Co-administration is contraindicated with these anticonvulsants. At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
| Antimycobacterials | ||
| rifampin†
rifapentine | ↓ doravirine | Co-administration is contraindicated with rifampin or rifapentine.
At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
| rifabutin† | ↓ doravirine | Increase PIFELTRO dosage to one tablet twice daily when co-administered with rifabutin [see Dosage and Administration (2.2)]. |
| Cytotoxic Agents | ||
| mitotane | ↓ doravirine | Co-administration is contraindicated with mitotane. At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
| HIV Antiviral Agents | ||
| efavirenz†
etravirine nevirapine | ↓ doravirine | Use with efavirenz, etravirine, or nevirapine is not recommended. |
| Herbal Products | ||
| St. John's wort | ↓ doravirine | Co-administration is contraindicated with St. John's wort. At least a 4-week cessation period is recommended prior to initiation of PIFELTRO. |
No clinically significant changes in concentration were observed for doravirine when co-administered with the following agents: dolutegravir, TDF, lamivudine, elbasvir and grazoprevir, ledipasvir and sofosbuvir, ritonavir, ketoconazole, aluminum hydroxide/magnesium hydroxide/simethicone containing antacid, pantoprazole, and methadone [see Clinical Pharmacology (12.3)].
8. Use In Specific Populations
8.4 Pediatric Use
The safety and efficacy of PIFELTRO for the treatment of HIV-1 infection have been established in pediatric patients weighing at least 35 kg [see Indications and Usage (1) and Dosage and Administration (2.1)].
Use of PIFELTRO in this group is supported by evidence from adequate and well-controlled trials in adults and an open-label trial in virologically-suppressed or treatment-naïve pediatric subjects 12 to less than 18 years of age. The safety, efficacy, and exposure of doravirine in these pediatric subjects were similar to that in adults. [see Adverse Reactions (6.1), Clinical Pharmacology (12.3), and Clinical Studies (14.3).]
Safety and efficacy of PIFELTRO in pediatric patients weighing less than 35 kg have not been established.
8.5 Geriatric Use
Clinical trials of PIFELTRO did not include sufficient numbers of subjects aged 65 years and over to determine whether they respond differently from younger subjects. In general, caution should be exercised in the administration of PIFELTRO in elderly patients, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy [see Clinical Pharmacology (12.3)].
11. Pifeltro Description
PIFELTRO is a film-coated tablet containing doravirine for oral administration.
Doravirine is an HIV-1 non-nucleoside reverse transcriptase inhibitor (NNRTI).
Each tablet contains 100 mg of doravirine as the active ingredient. The tablets include the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hypromellose acetate succinate, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. The tablets are film coated with a coating material containing the following inactive ingredients: hypromellose, lactose monohydrate, titanium dioxide, and triacetin. The coated tablets are polished with carnauba wax.
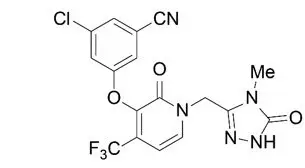
The chemical name for doravirine is 3-chloro-5-[[1-[(4,5-dihydro-4-methyl-5-oxo-1H-1,2,4-triazol-3-yl)methyl]-1,2-dihydro-2-oxo-4-(trifluoromethyl)-3-pyridinyl]oxy]benzonitrile.
It has a molecular formula of C17H11ClF3N5O3 and a molecular weight of 425.75.
It has the following structural formula:
Doravirine is practically insoluble in water.
12. Pifeltro - Clinical Pharmacology
12.2 Pharmacodynamics
In a Phase 2 trial evaluating doravirine over a dose range of 0.25 to 2 times the recommended dose of PIFELTRO, (in combination with FTC/TDF) in HIV-1-infected subjects with no antiretroviral treatment history, no exposure-response relationship for efficacy was identified for doravirine.
12.3 Pharmacokinetics
Doravirine pharmacokinetics are similar in healthy subjects and HIV-1-infected subjects. Doravirine pharmacokinetics are provided in Table 7.
| Parameter | Doravirine |
|---|---|
| Abbreviations: AUC=area under the time concentration curve; Cmax=maximum concentration; C24=concentration at 24 hours; Tmax time to Cmax; Vdss= volume of distribution at steady state, t1/2=elimination half-life; CL/F=apparent clearance; CLrenal=apparent renal clearance | |
|
|
| General | |
| Steady State Exposure*,† | |
| AUC0-24
(mcg∙h/mL) | 16.1 (29) |
| Cmax
(mcg/mL) | 0.962 (19) |
| C24
(mcg/mL) | 0.396 (63) |
| Time to Steady State (Days) | 2 |
| Accumulation Ratio | 1.2 to 1.4 |
| Absorption | |
| Absolute Bioavailability | 64% |
| Tmax (h) | 2 |
| Effect of Food‡ | |
| AUC Ratio | 1.16 (1.06, 1.26) |
| Cmax Ratio | 1.03 (0.89, 1.19) |
| C24 Ratio | 1.36 (1.19, 1.55) |
| Distribution | |
| Vdss (L)§ | 60.5 |
| Plasma Protein Binding | 76% |
| Elimination | |
| t1/2 (h) | 15 |
| CL/F (mL/min)† | 106 (35.2) |
| CLrenal (mL/min)† | 9.3 (18.6) |
| Metabolism | |
| Primary Pathway(s) | CYP3A |
| Excretion | |
| Major Route of Elimination | Metabolism |
| Urine (unchanged) | 6% |
| Biliary/Fecal (unchanged) | Minor |
Specific Populations
In adults, no clinically significant difference on the pharmacokinetics of doravirine were observed based on age (18 to 78 years of age), sex, and race/ethnicity, mild to severe renal impairment (creatinine clearance (CLcr) >15 mL/min, estimated by Cockcroft-Gault), or moderate hepatic impairment (Child-Pugh B). The pharmacokinetics of doravirine in patients with end-stage renal disease or undergoing dialysis, or severe hepatic impairment (Child-Pugh C) is unknown.
Patients with Hepatic Impairment
No clinically significant difference in the pharmacokinetics of doravirine was observed in subjects with moderate hepatic impairment (Child-Pugh score B) compared to subjects without hepatic impairment. Doravirine has not been studied in subjects with severe hepatic impairment (Child-Pugh score C) [see Use in Specific Populations (8.7)].
Pediatric Patients
Mean doravirine exposures were similar in 54 pediatric patients aged 12 to less than 18 years and weighing at least 35 kg who received doravirine or DELSTRIGO in IMPAACT 2014 (Protocol 027) relative to adults following administration of doravirine or DELSTRIGO (Table 8). For pediatric patients weighing ≥ 35 kg and < 45 kg who receive doravirine 100 mg or DELSTRIGO, the population pharmacokinetic model-predicted mean C24 of doravirine was comparable to that achieved in adults, whereas mean AUC0-24 and Cmax of doravirine were 25% and 36% higher than adult values, respectively. However, the predicted AUC0-24 and Cmax increases are not considered clinically significant.
| Parameter* | Doravirine† |
|---|---|
| Abbreviations: AUC=area under the time concentration curve; Cmax=maximum concentration; C24=concentration at 24 hours | |
|
|
| AUC0-24
(mcg∙h/mL) | 16.4 (24) |
| Cmax
(mcg/mL) | 1.03 (16) |
| C24
(mcg/mL) | 0.379 (42) |
14. Clinical Studies
14.2 Clinical Trial Results in Virologically-Suppressed Adults
The efficacy of switching from a baseline regimen consisting of two NRTIs in combination with a PI plus either ritonavir or cobicistat, or elvitegravir plus cobicistat, or an NNRTI to DELSTRIGO was evaluated in a randomized, open-label trial (DRIVE-SHIFT, NCT02397096), in virologically-suppressed HIV-1-infected adults. Subjects must have been virologically-suppressed (HIV-1 RNA < 50 copies/mL) on their baseline regimen for at least 6 months prior to trial entry, with no history of virologic failure. Subjects were randomized to either switch to DELSTRIGO at baseline (n = 447, Immediate Switch Group (ISG)), or stay on their baseline regimen until Week 24, at which point they switched to DELSTRIGO (n = 223, Delayed Switch Group (DSG)).
At baseline, the median age of subjects was 43 years, 16% were female, and 24% were Non-White, 21% were of Hispanic or Latino ethnicity, 3% had hepatitis B and/or C virus co-infection, 17% had a history of AIDS, 96% had CD4+ T-cell count greater than or equal to 200 cells/mm3, 70% were on a regimen containing a PI plus ritonavir, 24% were on a regimen containing an NNRTI, 6% were on a regimen containing elvitegravir plus cobicistat, and 1% were on a regimen containing a PI plus cobicistat; these characteristics were similar between treatment groups.
Virologic outcome results are shown in Table 11.
| Outcome | DELSTRIGO Once Daily ISG Week 48 N=447 | Baseline Regimen DSG Week 24 N=223 |
|---|---|---|
|
||
| HIV-1 RNA ≥ 50 copies/mL* | 2% | 1% |
| ISG-DSG, Difference (95% CI) †‡ | 0.7% (-1.3%, 2.6%) | |
| HIV-1 RNA <50 copies/mL | 91% | 95% |
| No Virologic Data Within the Time Window | 8% | 4% |
| Discontinued study due to AE or Death§ | 3% | <1% |
| Discontinued study for Other Reasons¶ | 4% | 4% |
| On study but missing data in window | 0 | 0 |
| Proportion (%) of Subjects With HIV-1 RNA <50 copies/mL by Baseline and Demographic Category | ||
| Age (years) | ||
| < 50 | 90% (N = 320) | 95% (N = 157) |
| ≥ 50 | 94% (N = 127) | 94% (N = 66) |
| Gender | ||
| Male | 91% (N = 372) | 94% (N = 194) |
| Female | 91% (N = 75) | 100% (N = 29) |
| Race | ||
| White | 90% (N = 344) | 95% (N = 168) |
| Non-White | 93% (N = 103) | 93% (N = 55) |
| Ethnicity | ||
| Hispanic or Latino | 88% (N = 99) | 91% (N = 45) |
| Not Hispanic or Latino | 91% (N = 341) | 95% (N = 175) |
| CD4+ T-cell Count (cells/mm3) | ||
| <200 cells/mm3 | 85% (N = 13) | 75% (N = 4) |
| ≥200 cells/mm3 | 91% (N = 426) | 95% (N = 216) |
| Baseline Regimen# | ||
| PI plus either ritonavir or cobicistat | 90% (N=316) | 94% (N=156) |
| elvitegravir plus cobicistat or NNRTI | 93% (N=131) | 96% (N=67) |
14.3 Clinical Trial Results in Pediatric Patients
The efficacy of DELSTRIGO (DOR/3TC/TDF) was evaluated in cohort 2 of an open-label, single-arm 2-cohort trial in HIV-1-infected pediatric patients 12 to less than 18 years of age (IMPAACT 2014 (Protocol 027), NCT03332095). In cohort 1, virologically-suppressed subjects (n=9) received a single 100 mg dose of PIFELTRO followed by intensive PK sampling. In cohort 2, virologically-suppressed subjects (n=43) were switched to DELSTRIGO and treatment-naïve subjects (n=2) were started on DELSTRIGO.
In cohort 2, at baseline the median age of subjects was 15 years (range: 12 to 17), the median weight was 52 kg (range: 45 to 80), 58% were female, 78% were Asian and 22% were Black, and the median CD4+ T-cell count was 713 cells per mm3 (range 84 to 1397). After switching to DELSTRIGO, 95% (41/43) of virologically-suppressed subjects remained suppressed (HIV-1 RNA <50 copies/mL) at Week 24. One of the two treatment-naïve subjects achieved HIV-1 RNA <50 copies/mL at Week 24. The other treatment-naïve subject met the protocol-defined virologic failure criteria (defined as 2 consecutive plasma HIV-1 RNA test results ≥200 copies/mL at or after Week 24) and was evaluated for the development of resistance; no emergence of genotypic or phenotypic resistance to doravirine, lamivudine, or tenofovir was detected.
16. How is Pifeltro supplied
Each PIFELTRO tablet contains 100 mg of doravirine, is white, oval-shaped and film-coated, and is debossed with the corporate logo and 700 on one side and plain on the other side. Each bottle contains 30 tablets (NDC 0006-3069-01) with silica gel desiccant and is closed with a child-resistant closure.
| PIFELTRO
doravirine tablet, film coated |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Merck Sharp & Dohme LLC (118446553) |