Drug Detail:Glimepiride and pioglitazone (Glimepiride and pioglitazone [ glye-mep-ir-ide-and-pye-oh-gli-ta-zone ])
Drug Class: Antidiabetic combinations
Highlights of Prescribing Information
Pioglitazone and Glimepiride Tablets, for oral use
Initial U.S. Approval: 2006
WARNING: CONGESTIVE HEART FAILURE
See full prescribing information for complete boxed warning.
- Thiazolidinediones, including pioglitazone, which is a component of pioglitazone and glimepiride tablets, cause or exacerbate congestive heart failure in some patients. (5.1)
- After initiation of pioglitazone and glimepiride tablets, and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone and glimepiride tablets must be considered. (5.1)
- Pioglitazone and glimepiride tablets are not recommended in patients with symptomatic heart failure. (5.1)
- Initiation of pioglitazone and glimepiride tablets in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated. (4, 5.1)
Recent Major Changes
| Warnings and Precautions | |
| Urinary Bladder Tumors (5.6) | 12/2016 |
Indications and Usage for Pioglitazone and Glimepiride Tablets
Pioglitazone and glimepiride tablets are a thiazolidinedione and a sulfonylurea combination product indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus when treatment with both pioglitazone and glimepiride is appropriate. (1)
Important Limitations of Use:
- Not for treatment of type 1 diabetes or diabetic ketoacidosis. (1)
Pioglitazone and Glimepiride Tablets Dosage and Administration
- Individualize the starting dose of pioglitazone and glimepiride tablets based on the patient's current regimen. (2.1)
- May adjust the dosing based on effectiveness and tolerability while not exceeding the maximum recommended daily dose of pioglitazone 45 mg and glimepiride 8 mg. (2.1)
- Pioglitazone and glimepiride tablets should be given in a single dose once daily with meals. (2.1)
- Obtain liver tests before starting pioglitazone and glimepiride tablets. If abnormal, use caution when treating with pioglitazone and glimepiride tablets, investigate the probable cause, treat (if possible) and follow appropriately. Monitoring liver tests while on pioglitazone and glimepiride tablets is not recommended in patients without liver disease. (5.5)
Dosage Forms and Strengths
- Tablets: 30 mg pioglitazone/2 mg glimepiride. (3)
- Tablets: 30 mg pioglitazone/4 mg glimepiride. (3)
Contraindications
- Initiation in patients with established New York Heart Association (NYHA) Class III or IV heart failure [see Boxed Warning]. (4)
- Use in patients with known hypersensitivity to pioglitazone, glimepiride or any other component of pioglitazone and glimepiride tablets. (4)
- Hypersensitivity to sulfonamide derivatives. (4)
Warnings and Precautions
- Congestive heart failure: Fluid retention may occur and can exacerbate or lead to congestive heart failure. Combination use with insulin and use in congestive heart failure NYHA Class I and II may increase risk. Monitor patients for signs and symptoms. (5.1)
- Hypoglycemia: May be severe. When insulin or an insulin secretagogue is used with pioglitazone, a lower dose of the insulin or insulin secretagogue may be needed to reduce the risk of hypoglycemia. (5.2)
- Hypersensitivity Reactions: Postmarketing reports for glimepiride, a component of pioglitazone and glimepiride tablets, include anaphylaxis, angioedema and Stevens-Johnson Syndrome. Promptly discontinue pioglitazone and glimepiride tablets, assess for other cases, institute appropriate monitoring and treatment, and initiate alternative treatment for diabetes. (5.3)
- Potential increased risk of cardiovascular mortality with sulfonylureas: Inform patients of risk, benefits, and treatment alternatives. (5.4)
- Hepatic effects: Postmarketing reports of hepatic failure, sometimes fatal. Causality cannot be excluded. If liver injury is detected, promptly interrupt pioglitazone and glimepiride tablets and assess patient for probable cause, then treat cause if possible, to resolution or stabilization. Do not restart pioglitazone and glimepiride tablets if liver injury is confirmed and no alternate etiology can be found. (5.5)
- Bladder cancer: May increase the risk of bladder cancer. Do not use in patients with active bladder cancer. Use caution when using in patients with a prior history of bladder cancer. (5.6)
- Edema: Dose-related edema may occur. (5.7)
- Fractures: Increased incidence in female patients. Apply current standards of care for assessing and maintaining bone health. (5.8)
- Hemolytic anemia: Can occur if glucose 6-phosphate dehydrogenase (GP6D) deficient. Use with caution in patients with GP6D deficiency. (5.9)
- Macular edema: Postmarketing reports. Recommend regular eye exams in all patients with diabetes according to current standards of care with prompt evaluation for acute visual changes. (5.10)
- Macrovascular outcomes: There have been no clinical studies establishing conclusive evidence of macrovascular risk reduction with pioglitazone and glimepiride tablets. (5.11)
Adverse Reactions/Side Effects
Most common adverse reactions (≥5%) are upper respiratory tract infection, accidental injury, and combined edema/peripheral edema. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Takeda Pharmaceuticals at 1-877-825-3327 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Strong CYP2C8 inhibitors (e.g., gemfibrozil) increase pioglitazone concentrations. Limit pioglitazone dose to 15 mg daily. (2.3, 7.1)
- CYP2C8 inducers (e.g., rifampin) may decrease pioglitazone concentrations. (7.2)
- Topiramate may decrease pioglitazone concentrations. (7.3)
- Certain medications may affect glucose metabolism, requiring pioglitazone and glimepiride tablet dose adjustment and close monitoring of blood glucose. (7.4)
- Miconazole: Severe hypoglycemia can occur when pioglitazone and glimepiride tablets and oral miconazole are used concomitantly. (7.5)
- CYP2C9 interactions: Inhibitors and inducers may affect glycemic control by altering glimepiride plasma concentrations. (7.6)
- Colesevelam: Coadministration may reduce glimepiride absorption. Pioglitazone and glimepiride tablets should be administered at least 4 hours prior to colesevelam. (2.4, 7.7)
Use In Specific Populations
- Females and Males of Reproductive Potential: Advise premenopausal females of the potential for an unintended pregnancy. (8.3)
- Pediatrics: Not recommended for use in pediatric patients. (8.4)
- Geriatric or renally impaired patients: At risk for hypoglycemia with pioglitazone and glimepiride tablets. Use caution in dose selection and titration, and monitor closely. (8.5, 8.6)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 12/2017
Full Prescribing Information
WARNING: CONGESTIVE HEART FAILURE
- Thiazolidinediones, including pioglitazone, which is a component of pioglitazone and glimepiride tablets, cause or exacerbate congestive heart failure in some patients [see Warnings and Precautions (5.1)].
- After initiation of pioglitazone and glimepiride tablets and after dose increases, monitor patients carefully for signs and symptoms of heart failure (e.g., excessive, rapid weight gain, dyspnea, and/or edema). If heart failure develops, it should be managed according to current standards of care and discontinuation or dose reduction of pioglitazone and glimepiride tablets must be considered [see Warnings and Precautions (5.1)].
- Pioglitazone and glimepiride tablets are not recommended in patients with symptomatic heart failure [see Warnings and Precautions (5.1)].
- Initiation of pioglitazone and glimepiride tablets in patients with established New York Heart Association (NYHA) Class III or IV heart failure is contraindicated [see Contraindications (4) and Warnings and Precautions (5.1)].
1. Indications and Usage for Pioglitazone and Glimepiride Tablets
Pioglitazone and glimepiride tablets are indicated as an adjunct to diet and exercise to improve glycemic control in adults with type 2 diabetes mellitus who are already treated with a thiazolidinedione and sulfonylurea or who have inadequate glycemic control on a thiazolidinedione alone or a sulfonylurea alone [see Clinical Studies (14)].
2. Pioglitazone and Glimepiride Tablets Dosage and Administration
2.1 Recommendations for All Patients
Pioglitazone and glimepiride tablets should be taken once daily with the first main meal.
Pioglitazone and glimepiride tablets are available as a 30 mg pioglitazone plus 2 mg glimepiride or a 30 mg pioglitazone plus 4 mg glimepiride tablet. If therapy with a combination tablet containing pioglitazone and glimepiride is considered appropriate the recommended starting dose is:
- 30 mg/2 mg or 30 mg/4 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients inadequately controlled on glimepiride monotherapy: 30 mg/2 mg or 30 mg/4 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients inadequately controlled on pioglitazone monotherapy: 30 mg/2 mg once daily and gradually titrated, as needed, after assessing adequacy of therapeutic response and tolerability,
- for patients who are changing from combination therapy of pioglitazone plus glimepiride as separate tablets: pioglitazone and glimepiride tablets should be taken at doses that are as close as possible to the dose of pioglitazone and glimepiride already being taken,
- for patients currently on a different sulfonylurea monotherapy or switching from combination therapy of pioglitazone plus a different sulfonylurea (e.g., glyburide, glipizide, chlorpropamide, tolbutamide, acetohexamide): 30 mg/2 mg once daily and adjusted after assessing adequacy of therapeutic response. Observe for hypoglycemia for one to two weeks due to the potential overlapping drug effect.
- for patients with systolic dysfunction, the lowest approved dose of pioglitazone and glimepiride tablets should be prescribed only after titration from 15 mg to 30 mg of pioglitazone has been safely tolerated.
After initiation of pioglitazone and glimepiride tablets or with dose increase, monitor patients carefully for hypoglycemia and adverse reactions related to fluid retention such as weight gain, edema, and signs and symptoms of congestive heart failure [see Boxed Warning and Warnings and Precautions (5.7)].
Liver tests (serum alanine and aspartate aminotransferases, alkaline phosphatase, and total bilirubin) should be obtained prior to initiating pioglitazone and glimepiride tablets. Routine periodic monitoring of liver tests during treatment with pioglitazone and glimepiride tablets is not recommended in patients without liver disease. Patients who have liver test abnormalities prior to initiation of pioglitazone and glimepiride tablets or who are found to have abnormal liver tests while taking pioglitazone and glimepiride tablets should be managed as described under Warnings and Precautions [see Warnings and Precautions (5.5) and Clinical Pharmacology (12.3)].
2.2 Concomitant Use with an Insulin Secretagogue or Insulin
If hypoglycemia occurs in a patient coadministered pioglitazone and glimepiride tablets and an insulin secretagogue, the dose of the insulin secretagogue should be reduced.
If hypoglycemia occurs in a patient coadministered pioglitazone and glimepiride tablets and insulin, the dose of insulin should be decreased by 10% to 25%. Further adjustments to the insulin dose should be individualized based on glycemic response.
2.3 Concomitant Use with Strong CYP2C8 Inhibitors
Coadministration of pioglitazone and gemfibrozil, a strong CYP2C8 inhibitor, increases pioglitazone exposure approximately 3 fold. Therefore, the maximum recommended dose of pioglitazone is 15 mg daily when used in combination with gemfibrozil or other strong CYP2C8 inhibitors. If gemfibrozil or other CYP2C8 inhibitors need to co-administered, patients should switch to individual components of pioglitazone and glimepiride tablets because the minimum dose of pioglitazone in pioglitazone and glimepiride tablets exceeds 15 mg [see Drug Interactions (7.1) and Clinical Pharmacology (12.3)].
2.4 Concomitant Use with Colesevelam
When colesevelam is coadministered with glimepiride, maximum plasma concentration and total exposure to glimepiride is reduced. Therefore, pioglitazone and glimepiride tablets should be administered at least four hours prior to colesevelam [see Drug Interactions (7.7) and Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
- 30 mg/2 mg tablet: White to off-white, round, convex tablets, debossed with "4833G" on one side and "30/2" on the other
- 30 mg/4 mg tablet: White to off-white, round, convex tablets, debossed with "4833G" on one side and "30/4" on the other
4. Contraindications
- Initiation in patients with established NYHA Class III or IV heart failure [see Boxed Warning].
- Use in patients with known hypersensitivity to pioglitazone, glimepiride or any other component of pioglitazone and glimepiride tablets [see Warnings and Precautions (5.3)].
- Use in patients with known history of an allergic reaction to sulfonamide derivatives.
Reported hypersensitivity reactions with glimepiride include cutaneous eruptions with or without pruritus as well as more serious reactions (e.g., anaphylaxis, angioedema, Stevens-Johnson Syndrome, dyspnea) [see Warnings and Precautions (5.3) and Adverse Reactions (6.2)]
5. Warnings and Precautions
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed elsewhere in the labeling:
- Congestive Heart Failure [see Boxed Warning and Warnings and Precautions (5.1)]
- Hypoglycemia [see Warnings and Precautions (5.2)]
- Edema [see Warnings and Precautions (5.7)]
- Fractures [see Warnings and Precautions (5.8)]
- Hemolytic Anemia [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The adverse events reported in at least 5% of patients in the controlled 16 week clinical studies between placebo plus a sulfonylurea and pioglitazone (15 mg and 30 mg combined) plus sulfonylurea treatment arms were upper respiratory tract infection (15.5% and 16.6%), accidental injury (8.6% and 3.5%), and combined edema/peripheral edema (2.1% and 7.2%), respectively.
The incidence and type of adverse events reported in at least 5% of patients in any combined treatment group from the 24 week study comparing pioglitazone 30 mg plus a sulfonylurea and pioglitazone 45 mg plus a sulfonylurea are shown in Table 1; the rate of adverse events resulting in study discontinuation between the two treatment groups was 6% and 9.7%, respectively.
| Adverse Event | Pioglitazone 30 mg + Sulfonylurea N=351 n (%) | Pioglitazone 45 mg + Sulfonylurea N=351 n (%) |
|---|---|---|
| Hypoglycemia | 47 (13.4) | 55 (15.7) |
| Upper Respiratory Tract Infection | 43 (12.3) | 52 (14.8) |
| Weight Increased | 32 (9.1) | 47 (13.4) |
| Edema Lower Limb | 20 (5.7) | 43 (12.3) |
| Headache | 25 (7.1) | 14 (4.0) |
| Urinary Tract Infection | 20 (5.7) | 24 (6.8) |
| Diarrhea | 21 (6.0) | 15 (4.3) |
| Nausea | 18 (5.1) | 14 (4.0) |
| Pain in Limb | 19 (5.4) | 14 (4.0) |
In US double-blind studies, anemia was reported in ≤2% of patients treated with pioglitazone plus a sulfonylurea [see Warnings and Precautions (5.9)].
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of pioglitazone and glimepiride. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
7. Drug Interactions
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
Limited data with pioglitazone and glimepiride tablets or pioglitazone in pregnant women are not sufficient to determine a drug-associated risk for major birth defects or miscarriage. There are clinical considerations related to fetal and neonatal adverse reactions and drug discontinuation if glimepiride is used during pregnancy. There are risks to the mother and fetus associated with poorly controlled diabetes in pregnancy [see Clinical Considerations].
No adverse developmental effects were observed when pioglitazone was administered to pregnant rats and rabbits during organogenesis at exposures up to 5 and 35 times the 45 mg clinical dose, respectively, based on the body surface area. Administration of glimepiride to pregnant rats and rabbits during organogenesis induced maternal hypoglycemia and also increased fetal mortality at doses 50 (rats) and 0.1 times (rabbits) the 8 mg clinical dose, respectively, based on body surface area [see Data].
The estimated background risk of major birth defects is 6-10% in women with pre-gestational diabetes with a HbA1c >7 and has been reported to be as high as 20-25% in women with a HbA1c >10. The estimated background risk of miscarriage for the indicated population is unknown. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2-4% and 15-20% respectively.
Fetal/Neonatal Adverse Reaction
Neonates of women with gestational diabetes, who are treated with sulfonylureas during pregnancy, may be at increased risk for neonatal intensive care unit admission, and may develop respiratory distress, hypoglycemia, birth injury, and be large for gestational age. Prolonged severe hypoglycemia, lasting 4-10 days, has been reported in neonates born to mothers receiving a sulfonylurea at the time of delivery and has been reported with the use of agents with a prolonged half-life. Observe newborns for symptoms of hypoglycemia and respiratory distress and manage accordingly.
8.2 Lactation
Data
During pre- and postnatal studies in rats, glimepiride was present in lactational milk and in serum of nursing rat pups. Offspring exposed to high levels of glimepiride during lactation developed skeletal abnormalities (shortening, thickening and bending of the humerus) during the postnatal period.
8.3 Females and Males of Reproductive Potential
Discuss the potential for unintended pregnancy with premenopausal women as therapy with pioglitazone, like other thiazolidinediones, may result in ovulation in some anovulatory women.
8.4 Pediatric Use
Safety and effectiveness of pioglitazone and glimepiride tablets in pediatric patients have not been established.
Pioglitazone and glimepiride tablets are not recommended for use in pediatric patients based on adverse effects observed in adults, including fluid retention and congestive heart failure, fractures, and urinary bladder tumors [see Warnings and Precautions (5.1, 5.6, 5.7, 5.8)].
8.5 Geriatric Use
To minimize the risk of hypoglycemia, the initial dosing, dose increments, and maintenance dosage of pioglitazone and glimepiride tablets should be conservative. During initiation of pioglitazone and glimepiride tablet therapy and any subsequent dose adjustments, geriatric patients should be observed carefully for hypoglycemia.
8.6 Renal Impairment
To minimize the risk of hypoglycemia, the initial dosing, dose increments and maintenance dosage of pioglitazone and glimepiride tablets should be conservative. During initiation of pioglitazone and glimepiride tablet therapy and any subsequent dose adjustments, these patients should be observed carefully for hypoglycemia.
A multiple-dose titration study was conducted in 16 patients with type 2 diabetes and renal impairment using doses ranging from 1 mg to 8 mg daily for three months. Baseline creatinine clearance ranged from 10 to 60 mL/min. The pharmacokinetics of glimepiride were evaluated in the multiple-dose titration study and the results were consistent with those observed in patients enrolled in a single-dose study. In both studies, the relative total clearance of glimepiride increased when kidney function was impaired. Both studies also demonstrated that the elimination of the two major metabolites was reduced in patients with renal impairment [see Clinical Pharmacology (12.3)].
11. Pioglitazone and Glimepiride Tablets Description
Pioglitazone and glimepiride tablets are a thiazolidinedione and a sulfonylurea combination product that contains two oral antihyperglycemic agents: pioglitazone and glimepiride. The concomitant use of pioglitazone and a sulfonylurea, the class of drugs that includes glimepiride, has been previously approved based on clinical trials in patients with type 2 diabetes inadequately controlled on a sulfonylurea. Additional efficacy and safety information about pioglitazone and glimepiride monotherapies may be found in the prescribing information for each individual drug.
Pioglitazone is an oral antidiabetic medication.
Pioglitazone [(±)-5-[[4-[2-(5-ethyl-2-pyridinyl)ethoxy]phenyl]methyl]-2,4-] thiazolidinedione monohydrochloride contains one asymmetric carbon, and the compound is synthesized and used as the racemic mixture. The two enantiomers of pioglitazone interconvert in vivo. No differences were found in the pharmacologic activity between the two enantiomers. The structural formula is as shown:
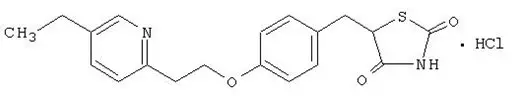
pioglitazone hydrochloride
Pioglitazone hydrochloride is an odorless, white crystalline powder that has a molecular formula of C19H20N2O3S∙HCl and a molecular weight of 392.90 daltons. It is soluble in N,N-dimethylformamide, slightly soluble in anhydrous ethanol, very slightly soluble in acetone and acetonitrile, practically insoluble in water, and insoluble in ether.
Glimepiride is an oral sulfonylurea chemically identified as 1-[[p-[2-(3-ethyl-4-methyl-2-oxo-3-pyrroline-1-carboxamido)ethyl]phenyl]sulfonyl]-3-(trans-4-methylcyclohexyl)-urea (C24H34N4O5S) with a molecular weight of 490.62. Glimepiride is a white to yellowish-white, crystalline, odorless to practically odorless powder and is practically insoluble in water. The structural formula is:

glimepiride
Pioglitazone and glimepiride tablets are available as a tablet for oral administration containing 30 mg pioglitazone (as the base) with 2 mg glimepiride (30 mg/2 mg) or 30 mg pioglitazone (as the base) with 4 mg glimepiride (30 mg/4 mg) formulated with the following excipients: croscarmellose sodium NF, lactose monohydrate NF, magnesium stearate NF, hydroxypropyl cellulose NF, polysorbate 80 NF, and microcrystalline cellulose NF.
12. Pioglitazone and Glimepiride Tablets - Clinical Pharmacology
12.1 Mechanism of Action
Pioglitazone and glimepiride tablets combine 2 antihyperglycemic agents with different mechanisms of action to improve glycemic control in patients with type 2 diabetes: pioglitazone, a member of the thiazolidinedione class, and glimepiride, a member of the sulfonylurea class. Thiazolidinediones are insulin-sensitizing agents that act primarily by enhancing peripheral glucose utilization, whereas sulfonylureas are insulin secretagogues that act primarily by stimulating release of insulin from functioning pancreatic beta cells.
12.3 Pharmacokinetics
Drug-Drug Interactions
Coadministration of pioglitazone (45 mg) and a sulfonylurea (5 mg glipizide) administered orally once daily for seven days did not alter the steady-state pharmacokinetics of glipizide. Glimepiride and glipizide have similar metabolic pathways and are mediated by CYP2C9; therefore, drug-drug interaction between pioglitazone and glimepiride is considered unlikely. Specific pharmacokinetic drug interaction studies with pioglitazone and glimepiride tablets have not been performed, although such studies have been conducted with the individual pioglitazone and glimepiride components.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
No animal studies have been conducted with pioglitazone and glimepiride tablets. The following data are based on findings in studies performed with pioglitazone or glimepiride individually.
14. Clinical Studies
There have been no clinical efficacy studies conducted with pioglitazone and glimepiride tablets. However, the efficacy and safety of the separate components have been previously established. The coadministration of pioglitazone and a sulfonylurea, including glimepiride, has been evaluated for efficacy and safety in two clinical studies. These clinical studies established an added benefit of pioglitazone in glycemic control of patients with inadequately controlled type 2 diabetes while on sulfonylurea therapy. Bioequivalence of pioglitazone and glimepiride tablets with coadministered pioglitazone and glimepiride tablets was demonstrated at the 30 mg/2 mg and 30 mg/4 mg dosage strengths [see Clinical Pharmacology (12.3)].
Two clinical trials were conducted with pioglitazone in combination with a sulfonylurea. Both studies included patients with type 2 diabetes on any dose of a sulfonylurea, either alone or in combination with another antidiabetic agent. All other antidiabetic agents were withdrawn at least three weeks prior to starting study treatment.
In the first study, 560 patients were randomized to receive 15 mg or 30 mg of pioglitazone or placebo once daily for 16 weeks in addition to their current sulfonylurea regimen. Treatment with pioglitazone as add-on to sulfonylurea produced statistically significant improvements in HbA1c and FGP at endpoint compared to placebo add-on to sulfonylurea (Table 15).
| Placebo + Sulfonylurea | Pioglitazone 15 mg + Sulfonylurea | Pioglitazone 30 mg + Sulfonylurea | |
|---|---|---|---|
|
|||
| Total Population | |||
| HbA1c (%) | N=181 | N=176 | N=182 |
| Baseline (mean) | 9.9 | 10.0 | 9.9 |
| Change from baseline (adjusted mean*) | 0.1 | -0.8 | -1.2 |
| Difference from placebo + sulfonylurea (adjusted mean*) 95% Confidence Interval | -0.9†
(-1.2, -0.6) | -1.3†
(-1.6, -1.0) |
|
| Fasting Plasma Glucose (mg/dL) | N=182 | N=179 | N=186 |
| Baseline (mean) | 236 | 247 | 239 |
| Change from baseline (adjusted mean*) | 6 | -34 | -52 |
| Difference from placebo + sulfonylurea (adjusted mean*) 95% Confidence Interval | -39†
(-52, -27) | -58†
(-70, -46) |
|
In the second trial, 702 patients were randomized to receive 30 mg or 45 mg of pioglitazone once daily for 24 weeks in addition to their current sulfonylurea regimen. The mean reduction from baseline at Week 24 in HbA1c was 1.6% for the 30 mg dose and 1.7% for the 45 mg dose (see Table 16). The mean reduction from baseline at Week 24 in FPG was 52 mg/dL for the 30 mg dose and 56 mg/dL for the 45 mg dose.
The therapeutic effect of pioglitazone in combination with sulfonylurea was observed in patients regardless of the sulfonylurea dose.
| Pioglitazone 30 mg + Sulfonylurea | Pioglitazone 45 mg + Sulfonylurea | |
|---|---|---|
| 95% CI = 95% confidence interval | ||
|
||
| Total Population | ||
| HbA1c (%) | N=340 | N=332 |
| Baseline (mean) | 9.8 | 9.9 |
| Change from baseline (adjusted mean*) | -1.6 | -1.7 |
| Difference from 30 mg daily pioglitazone + sulfonylurea (adjusted mean*) (95% CI) | -0.1 (-0.4, 0.1) |
|
| Fasting Plasma Glucose (mg/dL) | N=338 | N=329 |
| Baseline (mean) | 214 | 217 |
| Change from baseline (adjusted mean*) | -52 | -56 |
| Difference from 30 mg daily pioglitazone + sulfonylurea (adjusted mean*) (95% CI) | -5 (-12, 3) |
|
16. How is Pioglitazone and Glimepiride Tablets supplied
Pioglitazone and glimepiride tablets are available in 30 mg pioglitazone plus 2 mg glimepiride or 30 mg pioglitazone plus 4 mg glimepiride tablets as follows:
30 mg/2 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/2 on the other, available in:
NDC 66993-821-30 Bottles of 30
30 mg/4 mg tablet: white to off-white, round, convex tablets, debossed with 4833G on one side and 30/4 on the other, available in:
NDC 66993-822-30 Bottles of 30
17. Patient Counseling Information
See FDA-approved patient labeling (Medication Guide).
- Inform patients that pioglitazone and glimepiride tablets are not recommended for patients with symptoms of heart failure.
- Inform patients that patients with severe heart failure (NYHA Class III or IV) cannot start pioglitazone and glimepiride tablets as the risks exceed the benefits in such patients.
- It is important to instruct patients to adhere to dietary instructions and to have blood glucose and glycosylated hemoglobin tested regularly. During periods of stress such as fever, trauma, infection, or surgery, medication requirements may change and patients should be reminded to seek medical advice promptly. Patients should also be informed of the potential risks and advantages of pioglitazone and glimepiride tablets and of alternative modes of therapy.
- Tell patients to promptly report any sign of macroscopic hematuria or other symptoms such as dysuria or urinary urgency that develop or increase during treatment as these may be due to bladder cancer.
- Prior to initiation of pioglitazone and glimepiride tablet therapy, the risks of hypoglycemia, its symptoms and treatment, and conditions that predispose to its development should be explained to patients and responsible family members [see Warnings and Precautions (5.2)]. Combination therapy of pioglitazone and glimepiride tablets with other antihyperglycemic agents may also cause hypoglycemia.
- Patients who experience an unusually rapid increase in weight or edema or who develop shortness of breath or other symptoms of heart failure while on pioglitazone and glimepiride tablets should immediately report these symptoms to a physician.
- Tell patients to promptly stop taking pioglitazone and glimepiride tablets and seek immediate medical advice if there is unexplained nausea, vomiting, abdominal pain, fatigue, anorexia, or dark urine as these symptoms may be due to hepatotoxicity.
- Inform female patients that treatment with pioglitazone, like other thiazolidinediones may result in an unintended pregnancy in some premenopausal anovulatory females due to its effect on ovulation [see Use in Specific Populations (8.3)].
- Patients should be told to take a single dose of pioglitazone and glimepiride tablets once daily with the first main meal and instructed that any change in dosing should be made only if directed by their physician [see Dosage and Administration (2)].
Medication Guide
Read this Medication Guide carefully before you start taking pioglitazone and glimepiride tablets and each time you get a refill. There may be new information. This information does not take the place of talking with your doctor about your medical condition or your treatment. If you have any questions about pioglitazone and glimepiride tablets, ask your doctor or pharmacist.
What is the most important information I should know about pioglitazone and glimepiride tablets?
Pioglitazone and glimepiride tablets can cause serious side effects, including new or worse heart failure.
- Pioglitazone, one of the medicines in pioglitazone and glimepiride tablets, can cause your body to keep extra fluid (fluid retention), which leads to swelling (edema) and weight gain. Extra body fluid can make some heart problems worse or lead to heart failure. Heart failure means your heart does not pump blood well enough
- Do not take pioglitazone and glimepiride tablets if you have severe heart failure
- If you have heart failure with symptoms (such as shortness of breath or swelling), even if these symptoms are not severe, pioglitazone and glimepiride tablets may not be right for you
Call your doctor right away if you have any of the following:
- swelling or fluid retention, especially in the ankles or legs
- shortness of breath or trouble breathing, especially when you lie down
- an unusually fast increase in weight
- unusual tiredness
Pioglitazone and glimepiride tablets can have other serious side effects. See "What are the possible side effects of pioglitazone and glimepiride tablets?"
What are pioglitazone and glimepiride tablets?
Pioglitazone and glimepiride tablets are a prescription medicine used with diet and exercise to improve blood sugar (glucose) control in adults with type 2 diabetes.
Pioglitazone and glimepiride tablets contain 2 prescription diabetes medicines called pioglitazone (ACTOS) and glimepiride, a sulfonylurea.
Pioglitazone and glimepiride tablets are not for people with type 1 diabetes.
Pioglitazone and glimepiride tablets are not for people with diabetic ketoacidosis (increased ketones in your blood or urine).
It is not known if pioglitazone and glimepiride tablets are safe and effective in children under the age of 18. Pioglitazone and glimepiride tablets are not recommended for use in children.
Who should not take pioglitazone and glimepiride tablets?
See "What is the most important information I should know about pioglitazone and glimepiride tablets?"
Do not take pioglitazone and glimepiride tablets if you:
- have severe heart failure
- are allergic to any of the ingredients in pioglitazone and glimepiride tablets. See the end of this Medication Guide for a complete list of ingredients in pioglitazone and glimepiride tablets
- have a condition called diabetic ketoacidosis. Diabetic ketoacidosis should be treated with insulin
Talk to your doctor before taking pioglitazone and glimepiride tablets if you have any of these conditions.
What should I tell my doctor before taking pioglitazone and glimepiride tablets?
Before you take pioglitazone and glimepiride tablets, tell your doctor if you:
- have heart failure
- have kidney problems
- have type 1 ("juvenile") diabetes or had diabetic ketoacidosis
- have a type of diabetic eye disease that causes swelling in the back of the eye (macular edema)
- have liver problems
- have or have had cancer of the bladder
- are pregnant or plan to become pregnant. It is not known if pioglitazone and glimepiride tablets can harm your unborn baby. Talk to your doctor if you are pregnant or plan to become pregnant about the best way to control your blood glucose levels while pregnant
- are a premenopausal woman (before the "change of life"), who does not have periods regularly or at all. Pioglitazone and glimepiride tablets may increase your chance of becoming pregnant. Talk to your doctor about birth control choices while taking pioglitazone and glimepiride tablets. Tell your doctor right away if you become pregnant while taking pioglitazone and glimepiride tablets
- are breastfeeding or plan to breastfeed. It is not known if pioglitazone and glimepiride tablets pass into your milk and if it can harm your baby. Talk to your doctor about the best way to control your blood glucose levels while breastfeeding
- have G6PD deficiency (an inherited condition where you don't produce enough of the enzyme [G6PD]). Taking glimepiride, one of the medicines in pioglitazone and glimepiride tablets, with this condition may cause your red blood cells to be destroyed too quickly (hemolytic anemia)
Tell your doctor about all the medicines you take including prescription and over the counter medicines, vitamins, and herbal supplements.
Pioglitazone and glimepiride tablets and some of your other medicines can affect each other. You may need to have your dose of pioglitazone and glimepiride tablets or certain other medicines changed.
Know the medicines you take. Keep a list of your medicines and show it to your doctor and pharmacist before you start a new medicine. They will tell you if it is okay to take pioglitazone and glimepiride tablets with other medicines.
How should I take pioglitazone and glimepiride tablets?
- Take pioglitazone and glimepiride tablets exactly as your doctor tells you to take them
- Your doctor may change your dose of pioglitazone and glimepiride tablets. Do not change your dose unless your doctor tells you to
- Pioglitazone and glimepiride tablets may be prescribed alone or with other diabetes medicines. This will depend on how well your blood sugar is controlled
- Take pioglitazone and glimepiride tablets one time each day with the first main meal
- If you take colesevelam, a medicine used to lower your cholesterol, take your pioglitazone and glimepiride tablets at least 4 hours before you take your colesevelam.
- If you miss a dose of pioglitazone and glimepiride tablets, take your next dose as prescribed unless your doctor tells you differently. Do not take two doses at one time the next day
- If you take too many pioglitazone and glimepiride tablets, call your doctor or go to the nearest hospital emergency room right away
- If your body is under stress such as from a fever, infection, accident, or surgery, the dose of your diabetes medicines may need to be changed. Call your doctor right away
- Stay on your diet and exercise programs and test your blood sugar regularly while taking pioglitazone and glimepiride tablets
- Your doctor should do certain blood tests before you start and while you take pioglitazone and glimepiride tablets
- Your doctor should also do hemoglobin A1C testing to check how well your blood sugar is controlled with pioglitazone and glimepiride tablets
- Your doctor should check your eyes regularly while you take pioglitazone and glimepiride tablets
What are the possible side effects of pioglitazone and glimepiride tablets?
Pioglitazone and glimepiride tablets may cause serious side effects including:
- See "What is the most important information I should know about pioglitazone and glimepiride tablets?"
- low blood sugar (hypoglycemia). This can happen if you skip meals, if you also use another medicine that lowers blood sugar, or if you have certain medical problems. Lightheadedness, dizziness, shakiness, or hunger may happen if your blood sugar is too low. Severe low blood sugar can cause unconsciousness (passing out), seizures, and death. Call your doctor if low blood sugar levels are a problem for you
-
liver problems. Call your doctor right away if you have:
- nausea or vomiting
- stomach pain
- unusual or unexplained tiredness
- loss of appetite
- dark urine
- yellowing of your skin or the whites of your eyes
-
bladder cancer. There may be an increased chance of having bladder cancer when you take pioglitazone and glimepiride tablets. You should not take pioglitazone and glimepiride tablets if you are receiving treatment for bladder cancer. Tell your doctor right away if you have any of the following symptoms of bladder cancer:
- blood or a red color in your urine
- an increased need to urinate
- pain while you urinate
- broken bones (fractures). Usually in the hand, upper arm, or foot in women. Talk to your doctor for advice on how to keep your bones healthy.
- diabetic eye disease with swelling in the back of the eye (macular edema). Tell your doctor right away if you have any changes in your vision. Your doctor should check your eyes regularly
- release of an egg from an ovary in a woman (ovulation) leading to pregnancy. Ovulation may happen when premenopausal women who do not have regular monthly periods take pioglitazone and glimepiride tablets. This can increase your chance of getting pregnant
The most common side effects of pioglitazone and glimepiride tablets include:
- cold-like symptoms (upper respiratory tract infection)
- headache
- sinus infection
- diarrhea
- nausea
- muscle pain
- sore throat
Tell your doctor if you have any side effect that bothers you or that does not go away. These are not all the side effects of pioglitazone and glimepiride tablets. For more information, ask your doctor or pharmacist.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store pioglitazone and glimepiride tablets?
- Store pioglitazone and glimepiride tablets at 68°F to 77°F (20°C to 25°C). Keep pioglitazone and glimepiride tablets in the original container to protect from light
- Keep the pioglitazone and glimepiride tablets bottle tightly closed and keep tablets dry
- Keep pioglitazone and glimepiride tablets and all medicines out of the reach of children
General information about the safe and effective use of pioglitazone and glimepiride tablets
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use pioglitazone and glimepiride tablets for a condition for which it was not prescribed. Do not give pioglitazone and glimepiride tablets to other people, even if they have the same symptoms you have. It may harm them.
This Medication Guide summarizes the most important information about pioglitazone and glimepiride tablets. If you would like more information, talk with your doctor. You can ask your doctor or pharmacist for information about pioglitazone and glimepiride tablets that is written for healthcare professionals. For more information, call 1-877-825-3327.
What are the ingredients in pioglitazone and glimepiride tablets?
Active ingredients: pioglitazone and glimepiride
Inactive ingredients: croscarmellose sodium, lactose monohydrate, magnesium stearate, hydroxypropyl cellulose, polysorbate 80, and microcrystalline cellulose
This Medication Guide has been approved by the U.S. Food and Drug Administration.
Distributed by:
Prasco Laboratories
Mason, OH 45040 USA
Revised: December 2016
ACTOS is a registered trademark of Takeda Pharmaceutical Company Limited and used under license by Takeda Pharmaceuticals America, Inc.
PGL329 R3
| PIOGLITAZONE AND GLIMEPIRIDE
pioglitazone and glimepiride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| PIOGLITAZONE AND GLIMEPIRIDE
pioglitazone and glimepiride tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Prasco Laboratories (065969375) |
| Registrant - Takeda Pharmaceuticals America, Inc. (830134016) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Takeda GmbH | 313270015 | MANUFACTURE(66993-821, 66993-822) , ANALYSIS(66993-821, 66993-822) | |






