Drug Detail:Piqray (Alpelisib [ al-pel-i-sib ])
Drug Class: PI3K inhibitors
Highlights of Prescribing Information
PIQRAY® (alpelisib) tablets, for oral use
Initial U.S. Approval: 2019
Recent Major Changes
|
Dosage and Administration, Dose Modifications for Adverse Reactions (2.3) |
5/2022 |
|
Warnings and Precautions, Severe Hypersensitivity (5.1) |
5/2022 |
|
Warnings and Precautions, Diarrhea or Colitis (5.5) |
5/2022 |
Indications and Usage for Piqray
PIQRAY is a kinase inhibitor indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen. (1)
Piqray Dosage and Administration
- Recommended Dose: 300 mg (two 150 mg tablets) taken orally once daily with food. (2.2)
- For adverse reactions, consider dose interruption, dose reduction, or discontinuation. (2.3)
Dosage Forms and Strengths
Tablets: 50 mg, 150 mg, and 200 mg (3)
Contraindications
Severe hypersensitivity to PIQRAY or to any of its components. (4)
Warnings and Precautions
- Severe Hypersensitivity: Permanently discontinue PIQRAY. Promptly initiate appropriate treatment. (5.1)
- Severe Cutaneous Adverse Reactions (SCARs): PIQRAY can cause SCARs, including Stevens-Johnson syndrome (SJS), erythema multiforme (EM), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS). Interrupt PIQRAY for signs or symptoms of SCARs. Permanently discontinue PIQRAY if SCARs are confirmed. (5.2)
- Hyperglycemia: PIQRAY can cause severe hyperglycemia, in some cases associated with hyperglycemic hyperosmolar non-ketotic syndrome (HHNKS) or ketoacidosis. The safety of PIQRAY in patients with Type 1 or uncontrolled Type 2 diabetes has not been established. Before initiating treatment with PIQRAY, test fasting plasma glucose (FPG), HbA1c, and optimize blood glucose. After initiating treatment, monitor periodically. Initiate or optimize anti-hyperglycemic medications as clinically indicated. Interrupt, reduce dose, or discontinue PIQRAY if severe hyperglycemia occurs. (2.3, 5.3)
- Pneumonitis: PIQRAY can cause severe pneumonitis and interstitial lung disease. Monitor for clinical symptoms or radiological changes. Interrupt or discontinue PIQRAY if severe pneumonitis occurs. (2.3, 5.4)
-
Diarrhea or Colitis: PIQRAY causes diarrhea in most patients and may be severe, resulting in dehydration and acute kidney injury. Advise patients to start antidiarrheal treatment, increase oral fluids, and notify their healthcare provider if diarrhea occurs.
Colitis has been reported in patients treated with PIQRAY. Monitor for diarrhea and additional symptoms of colitis, including abdominal pain and mucus or blood in stool.
Interrupt, reduce dose, or discontinue PIQRAY if severe diarrhea or colitis occurs. Patients with colitis may require additional treatment, such as enteric-acting and/or systemic steroids. (2.3, 5.5, 6.2) - Embryo-Fetal Toxicity: PIQRAY can cause fetal harm. Advise patients of potential risk to a fetus and to use effective contraception. (5.6, 8.1, 8.3) Refer to the Full Prescribing Information of fulvestrant for pregnancy and contraception information.
Adverse Reactions/Side Effects
Most common adverse reactions, including laboratory abnormalities (all Grades, incidence ≥ 20%) were glucose increased, creatinine increased, diarrhea, rash, lymphocyte count decreased, gamma-glutamyl transferase (GGT) increased, nausea, alanine aminotransferase (ALT) increased, fatigue, hemoglobin decreased, lipase increased, decreased appetite, stomatitis, vomiting, weight decreased, calcium decreased, glucose decreased, activated partial thromboplastin time (aPTT) prolonged, and alopecia. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Novartis Pharmaceuticals Corporation at 1-888-669-6682 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- CYP3A4 Inducers: Avoid coadministration of PIQRAY with a strong CYP3A4 inducer. Consider an alternative concomitant drug with no or minimal potential to induce CYP3A4. (7.1)
- Breast Cancer Resistance Protein (BCRP) Inhibitors: Avoid the use of BCRP inhibitors in patients treated with PIQRAY. If unable to use alternative drugs, closely monitor for increased adverse reactions. (7.1)
- CYP2C9 Substrates: Closely monitor when PIQRAY is coadministered with CYP2C9 substrates where decreases in the plasma concentration of these drugs may reduce activity. (7.2)
Use In Specific Populations
Lactation: Advise not to breastfeed. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2022
Related/similar drugs
Kisqali, Keytruda, Trodelvy, tamoxifen, Arimidex, Femara, XelodaFull Prescribing Information
1. Indications and Usage for Piqray
PIQRAY is indicated in combination with fulvestrant for the treatment of postmenopausal women, and men, with hormone receptor (HR)-positive, human epidermal growth factor receptor 2 (HER2)-negative, PIK3CA-mutated, advanced or metastatic breast cancer as detected by an FDA-approved test following progression on or after an endocrine-based regimen.
2. Piqray Dosage and Administration
2.1 Patient Selection
Select patients for the treatment of HR-positive, HER2-negative advanced or metastatic breast cancer with PIQRAY, based on the presence of one or more PIK3CA mutations in tumor tissue or plasma specimens [see Clinical Studies (14)]. If no mutation is detected in a plasma specimen, test tumor tissue. Information on FDA-approved tests for the detection of PIK3CA mutations in breast cancer is available at: http://www.fda.gov/CompanionDiagnostics.
2.2 Dosage and Administration
The recommended dose of PIQRAY is 300 mg (two 150 mg film-coated tablets) taken orally, once daily, with food [see Clinical Pharmacology (12.3)].
Continue treatment until disease progression or unacceptable toxicity occurs [see Dosage and Administration (2.3)].
Patients should take their dose of PIQRAY at approximately the same time each day.
Swallow PIQRAY tablets whole (tablets should not be chewed, crushed or split prior to swallowing). No tablet should be ingested if it is broken, cracked, or otherwise not intact.
If a dose of PIQRAY is missed, it can be taken with food within 9 hours after the time it is usually taken. After more than 9 hours, skip the dose for that day. The next day, take PIQRAY at the usual time.
If the patient vomits after taking the dose, advise the patient not to take an additional dose on that day, and to resume the dosing schedule the next day at the usual time.
When given with PIQRAY, the recommended dose of fulvestrant is 500 mg administered on Days 1, 15, and 29, and once monthly thereafter. Refer to the Full Prescribing Information for fulvestrant.
2.3 Dose Modifications for Adverse Reactions
The recommended dose modifications for adverse reactions (ARs) are listed in Table 1.
| 1Only one dose reduction is permitted for pancreatitis. 2If further dose reduction below 200 mg once daily is required, discontinue PIQRAY. |
||
| PIQRAY Dose Level | Dose and Schedule | Number and Strength of Tablets |
| Starting dose | 300 mg once daily | Two 150 mg tablets |
| First-dose reduction | 250 mg once daily | One 200 mg tablet and one 50 mg tablet |
| Second-dose reduction | 200 mg once daily2 | One 200 mg tablet |
Tables 2, 3, 4, and 5 summarize recommendations for dose interruption, reduction, or discontinuation of PIQRAY in the management of specific ARs.
Cutaneous Adverse Reactions
If a severe cutaneous adverse reaction (SCAR) is confirmed, permanently discontinue PIQRAY. Do not reintroduce PIQRAY in patients who have experienced previous SCAR during PIQRAY treatment [see Warnings and Precautions (5.2)].
| 1Grading according to Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. 2For all grades of rash, consider consultation with a dermatologist. 3Antihistamines administered prior to rash onset may decrease incidence and severity of rash based on the SOLAR-1 trial. |
|
| [see Warnings and Precautions (5.1, 5.2)] | |
| Grade1,2 | Recommendation3 |
| Grade 1 (< 10% body surface area (BSA) with active skin toxicity) | No PIQRAY dose adjustment required. Initiate topical corticosteroid treatment. Consider adding oral antihistamine to manage symptoms. If active rash is not improved within 28 days of appropriate treatment, add a low dose systemic corticosteroid. If the etiology is SCAR, permanently discontinue PIQRAY. |
| Grade 2 (10%-30% BSA with active skin toxicity) | No PIQRAY dose adjustment required. Initiate or intensify topical corticosteroid and oral antihistamine treatment. Consider low dose systemic corticosteroid treatment. If rash improves to Grade ≤ 1 within 10 days, systemic corticosteroid may be discontinued. If the etiology is SCAR, permanently discontinue PIQRAY. |
| Grade 3 (e.g., severe rash not responsive to medical management) (> 30% BSA with active skin toxicity) | Interrupt PIQRAY. Initiate or intensify topical/systemic corticosteroid and oral antihistamine treatment. If the etiology is SCAR, permanently discontinue PIQRAY. If the etiology is not a SCAR, interrupt dose until improvement to Grade ≤ 1, then resume PIQRAY at next lower dose level. |
| Grade 4 (e.g., severe bullous, blistering or exfoliating skin conditions) (any % BSA associated with extensive superinfection, with IV antibiotics indicated; life-threatening consequences) | Permanently discontinue PIQRAY. |
Hyperglycemia
Before initiating treatment with PIQRAY, test fasting plasma glucose (FPG), HbA1c, and optimize blood glucose. After initiating treatment with PIQRAY, monitor fasting glucose (FPG or fasting blood glucose) at least once every week for the first 2 weeks, then at least once every 4 weeks, and as clinically indicated. Monitor HbA1c every 3 months and as clinically indicated. In patients with risk factors for hyperglycemia, monitor fasting glucose more closely and as clinically indicated [see Warnings and Precautions (5.3)].
| Abbreviation: ULN, upper limit of normal. 1FPG/Fasting Blood Glucose/Grade levels reflect hyperglycemia grading according to Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. 2Initiate applicable anti-hyperglycemic medications, including metformin, SGLT2 inhibitors or insulin sensitizers (such as thiazolidinediones or dipeptidyl peptidase-4 inhibitors), and review respective prescribing information for dosing and dose titration recommendations, including local hyperglycemic treatment guidelines. Metformin was recommended in the SOLAR-1 trial with the following guidance: Initiate metformin 500 mg once daily. Based on tolerability, metformin dose may be increased to 500 mg twice daily, followed by 500 mg with breakfast, and 1,000 mg with dinner, followed by further increase to 1,000 mg twice daily if needed [see Warnings and Precautions (5.3)]. 3As recommended in the SOLAR-1 trial, insulin may be used for 1-2 days until hyperglycemia resolves. However, this may not be necessary in the majority of PIQRAY-induced hyperglycemia, given the short half-life of PIQRAY and the expectation of glucose levels normalizing after interruption of PIQRAY. |
|
| [see Warnings and Precautions (5.3)] | |
| Fasting Plasma Glucose (FPG)/Fasting Blood Glucose Values1 | Recommendation |
| Dose modifications and management should only be based on fasting glucose values (FPG or fasting blood glucose). | |
| Grade 1 Fasting glucose > ULN -160 mg/dL or > ULN -8.9 mmol/L | No PIQRAY dose adjustment required. Initiate or intensify anti-hyperglycemic treatment2. |
| Grade 2 Fasting glucose > 160-250 mg/dL or > 8.9-13.9 mmol/L | No PIQRAY dose adjustment required. Initiate or intensify anti-hyperglycemic treatment2. If fasting glucose does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 21 days under appropriate anti-hyperglycemic treatment2,3, reduce PIQRAY dose by 1 dose level and follow fasting glucose value specific recommendations. |
| Grade 3 > 250-500 mg/dL or > 13.9-27.8 mmol/L | Interrupt PIQRAY. Initiate or intensify oral anti-hyperglycemic treatment2 and consider additional anti-hyperglycemic medications3 for 1-2 days until hyperglycemia improves, as clinically indicated. Administer intravenous hydration and consider appropriate treatment (e.g., intervention for electrolyte/ketoacidosis/hyperosmolar disturbances). If fasting glucose decreases to ≤ 160 mg/dL or 8.9 mmol/L within 3 to 5 days under appropriate anti-hyperglycemic treatment, resume PIQRAY at 1 lower dose level. If fasting glucose does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 3 to 5 days under appropriate anti-hyperglycemic treatment, consultation with a physician with expertise in the treatment of hyperglycemia is recommended. If fasting glucose does not decrease to ≤ 160 mg/dL or 8.9 mmol/L within 21 days following appropriate anti-hyperglycemic treatment2,3, permanently discontinue PIQRAY treatment. |
| Grade 4 > 500 mg/dL or > 27.8 mmol/L | Interrupt PIQRAY. Initiate or intensify appropriate anti-hyperglycemic treatment2,3 (administer intravenous hydration and consider appropriate treatment (e.g., intervention for electrolyte/ketoacidosis/hyperosmolar disturbances)), re-check fasting glucose within 24 hours and as clinically indicated. If fasting glucose decreases to ≤ 500 mg/dL or 27.8 mmol/L, follow fasting glucose value-specific recommendations for Grade 3. If fasting glucose is confirmed at > 500 mg/dL or 27.8 mmol/L, permanently discontinue PIQRAY treatment. |
Diarrhea or Colitis
| 1Grading according to CTCAE Version 5.0. 2For Grade 2 and 3 colitis, consider additional treatment, such as enteric-acting and/or systemic steroids. |
|
| [see Warnings and Precautions (5.5)] | |
| Grade1 | Recommendation |
| Grade 1 | No PIQRAY dose adjustment is required. Initiate appropriate medical therapy and monitor as clinically indicated. |
| Grade 2 | Interrupt PIQRAY dose until improvement to Grade ≤ 1, then resume PIQRAY at the same dose level. For recurrent Grade ≥ 2, interrupt PIQRAY dose until improvement to Grade ≤ 1, then resume PIQRAY at the next lower dose level. Initiate or intensify appropriate medical therapy and monitor as clinically indicated2. |
| Grade 3 | Interrupt PIQRAY dose until improvement to Grade ≤ 1, then resume PIQRAY at the next lower dose level. Initiate or intensify appropriate medical therapy and monitor as clinically indicated2. |
| Grade 4 | Permanently discontinue PIQRAY. |
Other Toxicities
| 1Grading according to CTCAE Version 5.0. 2For Grade 2 and 3 pancreatitis, interrupt PIQRAY dose until improvement to Grade < 2 and resume at next lower-dose level. Only one dose reduction is permitted. If toxicity reoccurs, permanently discontinue PIQRAY treatment. 3For Grade 2 total bilirubin elevation, interrupt PIQRAY dose until improvement to Grade ≤ 1 and resume at the same dose if resolved in ≤ 14 days or resume at the next lower dose level if improved in > 14 days. |
|
| Grade1 | Recommendation |
| Grade 1 or 2 | No PIQRAY dose adjustment is required. Initiate appropriate medical therapy and monitor as clinically indicated2,3. |
| Grade 3 | Interrupt PIQRAY dose until improvement to Grade ≤ 1, then resume PIQRAY at the next lower dose level. |
| Grade 4 | Permanently discontinue PIQRAY. |
Refer to the Full Prescribing Information of fulvestrant for dose modification guidelines in the event of toxicity and for other relevant safety information.
3. Dosage Forms and Strengths
Tablets: 50 mg, 150 mg, and 200 mg alpelisib
50 mg: Light pink, unscored, round and curved with beveled edges film-coated tablet, imprinted with “L7” on one side and “NVR” on the other side.
150 mg: Pale red, unscored, ovaloid and curved with beveled edges film-coated tablet, imprinted with “UL7” on one side and “NVR” on the other side.
200 mg: Light red, unscored, ovaloid and curved with beveled edges film-coated tablet, imprinted with “YL7” on one side and “NVR” on the other side.
4. Contraindications
PIQRAY is contraindicated in patients with severe hypersensitivity to it or any of its components [see Warnings and Precautions (5.1)].
5. Warnings and Precautions
5.1 Severe Hypersensitivity
Severe hypersensitivity reactions, including anaphylaxis and anaphylactic shock, can occur in patients treated with PIQRAY. Severe hypersensitivity reactions were manifested by symptoms, including, but not limited to, dyspnea, flushing, rash, fever, or tachycardia.
The incidence of Grade 3 and 4 hypersensitivity reactions was 0.7% [see Adverse Reactions (6)].
Angioedema has been reported in the postmarketing setting in patients treated with PIQRAY [see Adverse Reactions (6.2)].
Advise patients of the signs and symptoms of severe hypersensitivity reactions. Permanently discontinue PIQRAY in the event of severe hypersensitivity.
5.2 Severe Cutaneous Adverse Reactions
Severe cutaneous adverse reactions (SCARs), including Stevens-Johnson syndrome (SJS), erythema multiforme (EM), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) can occur in patients treated with PIQRAY.
In the SOLAR-1 study, SJS and EM were reported in 0.4% and 1.1% of the patients, respectively [see Adverse Reactions (6.1)]. Drug reaction with eosinophilia and systemic symptoms (DRESS) was reported in patients treated with PIQRAY in the postmarketing setting [see Adverse Reactions (6.2)].
If signs or symptoms of SCARs occur, interrupt PIQRAY until the etiology of the reaction has been determined. Consultation with a dermatologist is recommended.
If a SCAR is confirmed, permanently discontinue PIQRAY. Do not reintroduce PIQRAY in patients who have experienced previous severe cutaneous adverse reactions during PIQRAY treatment.
If a SCAR is not confirmed, PIQRAY may require dose modifications, topical corticosteroids, or oral antihistamine treatment as described in Table 2 [see Dosage and Administration (2.3)].
Advise patients of the signs and symptoms of SCARs (e.g., a prodrome of fever, flu-like symptoms, mucosal lesions, progressive skin rash, or lymphadenopathy).
5.3 Hyperglycemia
Severe hyperglycemia, in some cases associated with hyperglycemic hyperosmolar non-ketotic syndrome (HHNKS) or ketoacidosis has occurred in patients treated with PIQRAY. Fatal cases of ketoacidosis have occurred in the postmarketing setting.
Hyperglycemia was reported in 65% of patients treated with PIQRAY. Grade 3 (FPG > 250 to 500 mg/dL) and Grade 4 (FPG > 500 mg/dL) hyperglycemia was reported in 33% and 3.9% of patients, respectively. Ketoacidosis was reported in 0.7% of patients (n = 2) treated with PIQRAY.
Among the patients who experienced Grade ≥ 2 (FPG 160 to 250 mg/dL) hyperglycemia, the median time to first occurrence of hyperglycemia was 15 days (range, 5 to 517 days).
In the 187 patients with hyperglycemia, 87% (163/187) were managed with anti-hyperglycemic medication, and 76% (142/187) reported use of metformin as single agent or in combination with other anti-hyperglycemic medication [i.e., insulin, dipeptidyl peptidase-4 (DPP-4) inhibitors, and sulfonylureas]. In patients with Grade ≥ 2 hyperglycemia with at least 1 grade improvement (n = 153), median time to improvement from the first event was 8 days (range, 2 to 65 days).
In all patients with elevated FPG who continued fulvestrant treatment after discontinuing PIQRAY (n = 54), 96% (n = 52) of patients had FPG levels that returned to baseline.
Before initiating treatment with PIQRAY, test fasting plasma glucose (FPG), HbA1c, and optimize blood glucose. After initiating treatment with PIQRAY, monitor fasting glucose (FPG or fasting blood glucose) at least once every week for the first 2 weeks, then at least once every 4 weeks, and as clinically indicated. Monitor HbA1c every 3 months and as clinically indicated. Monitor fasting glucose more frequently for the first few weeks during treatment with PIQRAY in patients with risk factors for hyperglycemia, such as obesity (BMI ≥ 30), elevated FPG, HbA1c at the upper limit of normal or above, use of concomitant systemic corticosteroids, or age ≥ 75 [see Use in Specific Populations (8.5)].
If a patient experiences hyperglycemia after initiating treatment with PIQRAY, monitor fasting glucose as clinically indicated, and at least twice weekly until fasting glucose decreases to normal levels. During treatment with anti-hyperglycemic medication, continue monitoring fasting glucose at least once a week for 8 weeks, followed by once every 2 weeks and as clinically indicated. Consider consultation with a healthcare practitioner with expertise in the treatment of hyperglycemia and counsel patients on lifestyle changes.
The safety of PIQRAY in patients with Type 1 and uncontrolled Type 2 diabetes has not been established as these patients were excluded from the SOLAR-1 trial. Patients with a medical history of controlled Type 2 diabetes were included. Patients with a history of diabetes mellitus may require intensified hyperglycemic treatment. Closely monitor patients with diabetes.
Based on the severity of the hyperglycemia, PIQRAY may require dose interruption, reduction, or discontinuation as described in Table 3 [see Dosage and Administration (2.3)].
Advise patients of the signs and symptoms of hyperglycemia (e.g., excessive thirst, urinating more often than usual or higher amount of urine than usual, or increased appetite with weight loss).
5.4 Pneumonitis
Severe pneumonitis, including acute interstitial pneumonitis and interstitial lung disease, can occur in patients treated with PIQRAY.
Pneumonitis was reported in 1.8% of patients treated with PIQRAY.
In patients who have new or worsening respiratory symptoms or are suspected to have developed pneumonitis, interrupt PIQRAY immediately and evaluate the patient for pneumonitis. Consider a diagnosis of non-infectious pneumonitis in patients presenting with non-specific respiratory signs and symptoms, such as hypoxia, cough, dyspnea, or interstitial infiltrates on radiologic exams and in whom infectious, neoplastic, and other causes have been excluded by means of appropriate investigations.
Permanently discontinue PIQRAY in all patients with confirmed pneumonitis.
Advise patients to immediately report new or worsening respiratory symptoms.
5.5 Diarrhea or Colitis
Severe diarrhea, resulting in dehydration and in some cases in acute kidney injury, can occur in patients treated with PIQRAY. Most patients (58%) experienced diarrhea during treatment with PIQRAY. Grade 3 diarrhea occurred in 7% (n = 19) of patients. Among patients with Grade 2 or 3 diarrhea (n = 71), the median time to onset was 46 days (range, 1 to 442 days).
In clinical trials, 63% of patients who experienced diarrhea required antidiarrheal medications (e.g., loperamide) to manage symptoms. Dose reductions of PIQRAY were required in 6% of patients, and 2.8% of patients permanently discontinued PIQRAY due to diarrhea.
Colitis has been reported in the postmarketing setting in patients treated with PIQRAY [see Adverse Reactions (6.2)].
Monitor patients for diarrhea and additional symptoms of colitis, such as abdominal pain and mucus or blood in stool. Based on the severity of the diarrhea or colitis, PIQRAY may require dose interruption, reduction, or discontinuation as described in Table 4 [see Dosage and Administration (2.3)].
Advise patients to start antidiarrheal treatment, increase oral fluids, and notify their healthcare provider if diarrhea occurs while taking PIQRAY.
Patients with colitis may require additional treatment, such as enteric-acting and/or systemic steroids.
5.6 Embryo-Fetal Toxicity
Based on findings in animals and its mechanism of action, PIQRAY can cause fetal harm when administered to a pregnant woman. In animal reproduction studies, oral administration of alpelisib to pregnant rats and rabbits during organogenesis caused adverse developmental outcomes, including embryo-fetal mortality (post-implantation loss), reduced fetal weights, and increased incidences of fetal malformations at maternal exposures based on area under the curve (AUC) that were ≥ 0.8 times the exposure in humans at the recommended dose of 300 mg/day. Advise pregnant women and females of reproductive potential of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception during treatment with PIQRAY and for 1 week after the last dose. Advise male patients with female partners of reproductive potential to use condoms and effective contraception during treatment with PIQRAY and for 1 week after the last dose [see Use in Specific Populations (8.1, 8.3) and Clinical Pharmacology (12.1)].
Refer to the Full Prescribing Information of fulvestrant for pregnancy and contraception information.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Severe Hypersensitivity [see Warnings and Precautions (5.1)]
- Severe Cutaneous Adverse Reactions [see Warnings and Precautions (5.2)]
- Hyperglycemia [see Warnings and Precautions (5.3)]
- Pneumonitis [see Warnings and Precautions (5.4)]
- Diarrhea or Colitis [see Warnings and Precautions (5.5)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
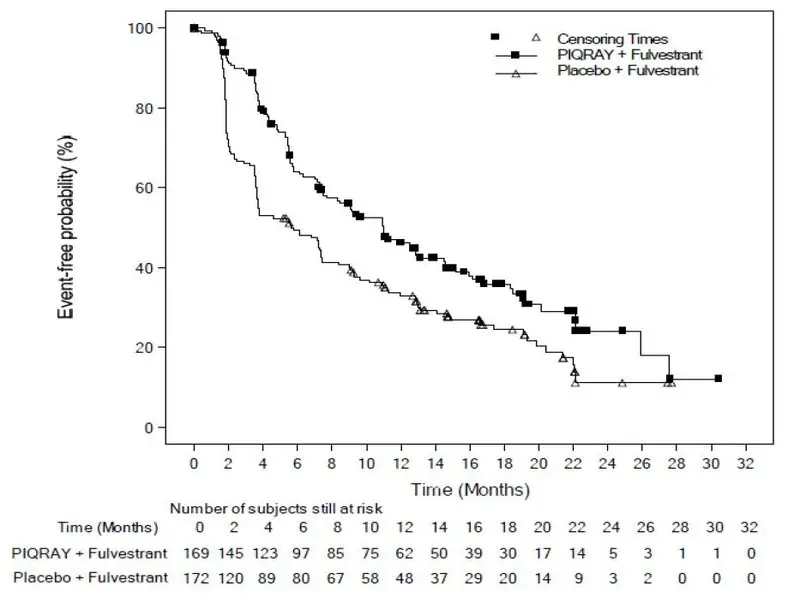
The safety of PIQRAY was evaluated in a randomized, double-blind, placebo-controlled trial (SOLAR-1) in 571 patients with HR-positive, HER2-negative, advanced or metastatic breast cancer enrolled into two cohorts, with or without a PIK3CA mutation [see Clinical Studies (14)].
Patients received either PIQRAY 300 mg plus fulvestrant (n = 284) or placebo plus fulvestrant (n = 287). Fulvestrant 500 mg was administered intramuscularly on Cycle 1, Day 1 and 15, and then at Day 1 of each 28-day cycle during treatment phase.
Two patients (0.7%) died while on treatment with PIQRAY plus fulvestrant due to causes other than the underlying malignancy. Causes of death included one cardio-respiratory arrest and one second primary malignancy. Neither was suspected to be related to study treatment.
Serious adverse reactions occurred in 35% of patients receiving PIQRAY plus fulvestrant. Serious adverse reactions in > 2% of patients receiving PIQRAY plus fulvestrant included hyperglycemia (10%), rash (3.5%), diarrhea (2.8%), acute kidney injury (2.5%), abdominal pain (2.1%), and anemia (2.1%).
Osteonecrosis of the jaw (ONJ) was reported in 4.2% of patients (12/284) in the PIQRAY plus fulvestrant arm compared to 1.4% of patients (4/287) in the placebo arm. All patients experiencing ONJ had prior or concomitant bisphosphonates or RANK-ligand inhibitor administration.
Among patients receiving PIQRAY plus fulvestrant, 4.6% permanently discontinued both PIQRAY and fulvestrant and 21% permanently discontinued PIQRAY alone, due to ARs. The most frequent ARs leading to treatment discontinuation of PIQRAY in > 2% patients receiving PIQRAY plus fulvestrant were hyperglycemia (6%), rash (4.2%), diarrhea (2.8%), and fatigue (2.5%).
Dose reductions due to ARs occurred in 55% of patients receiving PIQRAY plus fulvestrant. The most frequent ARs leading to dose reduction in > 2% patients receiving PIQRAY plus fulvestrant were hyperglycemia (29%), rash (9%), diarrhea (6%), stomatitis (3.5%), and mucosal inflammation (2.1%).
The most common adverse reactions, including laboratory abnormalities (all grades, incidence ≥ 20%) were glucose increased, creatinine increased, diarrhea, rash, lymphocyte count decreased, gamma-glutamyl transferase (GGT) increased, nausea, alanine aminotransferase (ALT) increased, fatigue, hemoglobin decreased, lipase increased, decreased appetite, stomatitis, vomiting, weight decreased, calcium decreased, glucose decreased, activated partial thromboplastin time (aPTT) prolonged, and alopecia.
Adverse reactions and laboratory abnormalities are listed in Table 6 and Table 7, respectively.
| Grading according to CTCAE Version 4.03. 1Stomatitis: including stomatitis, aphthous ulcer and mouth ulceration. 2Abdominal pain: abdominal pain, abdominal pain upper, abdominal pain lower. 3Fatigue: including fatigue, asthenia. 4Mucosal dryness: including dry mouth, mucosal dryness, vulvovaginal dryness. 5Urinary tract infection: including UTI and single case of urosepsis. 6Dysgeusia: including dysgeusia, ageusia, hypogeusia. 7Rash: including rash, rash maculo-papular, rash macular, rash generalized, rash papular, rash pruritic. 8Dry skin: including dry skin, skin fissures, xerosis, xeroderma. *No Grade 4 adverse reactions were reported. |
||||
| PIQRAY plus fulvestrant
N = 284 | Placebo plus fulvestrant
N = 287 |
|||
| Adverse reactions | All Grades | Grade 3-4 | All Grades | Grade 3-4 |
| % | % | % | % | |
| Gastrointestinal disorders | ||||
| Diarrhea | 58 | 7* | 16 | 0.3* |
| Nausea | 45 | 2.5* | 22 | 0.3* |
| Stomatitis1 | 30 | 2.5* | 6 | 0* |
| Vomiting | 27 | 0.7* | 10 | 0.3* |
| Abdominal pain2 | 17 | 1.4* | 11 | 1* |
| Dyspepsia | 11 | 0* | 6 | 0* |
| General disorders and administration site conditions | ||||
| Fatigue3 | 42 | 5* | 29 | 1* |
| Mucosal inflammation | 19 | 2.1* | 1 | 0* |
| Edema peripheral | 15 | 0* | 5 | 0.3* |
| Pyrexia | 14 | 0.7 | 4.9 | 0.3* |
| Mucosal dryness4 | 12 | 0.4* | 4.2 | 0* |
| Infections and infestations | ||||
| Urinary tract infection5 | 10 | 0.7* | 5 | 1* |
| Investigations | ||||
| Weight decreased | 27 | 3.9* | 2.1 | 0* |
| Metabolism and nutrition disorders | ||||
| Decreased appetite | 36 | 0.7* | 10 | 0.3* |
| Nervous system disorders | ||||
| Dysgeusia6 | 18 | 0.4* | 3.5 | 0* |
| Headache | 18 | 0.7* | 13 | 0* |
| Skin and subcutaneous tissue disorders | ||||
| Rash7 | 52 | 20* | 7 | 0.3* |
| Alopecia | 20 | 0* | 2.4 | 0* |
| Pruritus | 18 | 0.7* | 6 | 0* |
| Dry skin8 | 18 | 0.4* | 3.8 | 0* |
Among the patients with Grade 2 or 3 rash, the median time to first onset of Grade 2 or 3 rash was 12 days. A subgroup of 86 patients received prophylaxis, including antihistamines, prior to onset of rash. In these patients, rash was reported less frequently than in the overall population, for all grades rash (27% vs 54%), Grade 3 rash (12% vs 20%) and rash leading to permanent discontinuation of PIQRAY (3.5% vs 4.2%). Of the 153 patients who experienced rash, 141 had resolution of the rash.
| 1Glucose increase is an expected laboratory abnormality of PI3K inhibition. *No Grade 4 laboratory abnormalities were reported. |
||||
| PIQRAY plus fulvestrant
N = 284 | Placebo plus fulvestrant
N = 287 |
|||
| Laboratory Abnormality | All Grades | Grade 3-4 | All Grades | Grade 3-4 |
| % | % | % | % | |
| Hematological parameters | ||||
| Lymphocyte count decreased | 52 | 8 | 40 | 4.5* |
| Hemoglobin decreased | 42 | 4.2* | 29 | 1* |
| Activated Partial Thromboplastin Time (aPTT) prolonged | 21 | 0.7* | 16 | 0.3* |
| Platelet count decreased | 14 | 1.1 | 6 | 0* |
| Biochemical parameters | ||||
| Glucose increased1 | 79 | 39 | 34 | 1 |
| Creatinine increased | 67 | 2.8* | 25 | 0.7* |
| Gamma Glutamyl Transferase (GGT) increased | 52 | 11 | 44 | 10 |
| Alanine Aminotransferase (ALT) increased | 44 | 3.5 | 34 | 2.4* |
| Lipase increased | 42 | 7 | 25 | 6 |
| Calcium (corrected) decreased | 27 | 2.1 | 20 | 1.4 |
| Glucose decreased | 26 | 0.4 | 14 | 0* |
| Potassium decreased | 14 | 6 | 2.8 | 0.7* |
| Albumin decreased | 14 | 0* | 8 | 0* |
| Magnesium decreased | 11 | 0.4* | 4.2 | 0* |
6.2 Postmarketing Experience
The following adverse reactions have been identified during postapproval use of PIQRAY. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal disorders: Colitis
Metabolism and nutrition disorders: Hyperglycemic hyperosmolar nonketotic syndrome (HHNKS).
Skin and subcutaneous tissue disorders: Angioedema, Drug reaction with eosinophilia and systemic symptoms (DRESS).
7. Drug Interactions
7.1 Effect of Other Drugs on PIQRAY
CYP3A4 Inducer
Coadministration of PIQRAY with a strong CYP3A4 inducer decreases alpelisib concentration [see Clinical Pharmacology (12.3)], which may reduce alpelisib efficacy. Avoid concomitant use of strong CYP3A4 inducers and consider an alternative concomitant drug with no or minimal potential to induce CYP3A4.
Breast Cancer Resistance Protein Inhibitors
Coadministration of PIQRAY with a breast cancer resistance protein (BCRP) inhibitor may increase alpelisib concentration [see Clinical Pharmacology (12.3)], which may increase the risk of toxicities. Avoid the use of BCRP inhibitors in patients treated with PIQRAY. If unable to use alternative drugs, when PIQRAY is used in combination with BCRP inhibitors, closely monitor for increased adverse reactions.
7.2 Effect of PIQRAY on Other Drugs
CYP2C9 Substrates
Coadministration of PIQRAY with CYP2C9 substrates (e.g., warfarin) may reduce plasma concentration of these drugs [see Clinical Pharmacology (12.3)]. Closely monitor when PIQRAY is used in combination with CYP2C9 substrates where decreases in the plasma concentration of CYP2C9 substrates may reduce activity of these drugs.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
PIQRAY is used in combination with fulvestrant. Refer to the Full Prescribing Information of fulvestrant for pregnancy information.
Based on animal data and mechanism of action, PIQRAY can cause fetal harm when administered to a pregnant woman [see Clinical Pharmacology (12.1)]. There are no available data in pregnant women to inform the drug-associated risk. In animal reproduction studies, oral administration of alpelisib to pregnant rats and rabbits during organogenesis caused adverse developmental outcomes, including embryo-fetal mortality (post-implantation loss), reduced fetal weights, and increased incidences of fetal malformations at maternal exposures ≥ 0.8 times the exposure in humans based on AUC at the recommended dose of 300 mg/day (see Data). Advise pregnant women and females of reproductive potential of the potential risk to a fetus.
The estimated background risk of major birth defects and miscarriage for the indicated population is unknown. However, the estimated background risk of major birth defects is 2% to 4% and of miscarriage is 15% to 20% of clinically recognized pregnancies in the U.S. general population.
Data
Animal Data
In embryo-fetal development studies in rats and rabbits, pregnant animals received oral doses of alpelisib up to 30 mg/kg/day during the period of organogenesis.
In rats, oral administration of alpelisib resulted in maternal toxicity (body weight loss, low food consumption) and no viable fetuses (post-implantation loss) at 30 mg/kg/day (approximately 3 times the exposure in humans at the recommended dose of 300 mg/day based on AUC). At a dose of 10 mg/kg/day (approximately 0.8 times the exposure in humans at the recommended dose of 300 mg/day based on AUC), toxicities included reduced fetal weight and increased incidences of skeletal malformations (bent scapula and thickened or bent long bones) and fetal variations (enlarged brain ventricle, decreased bone ossification).
In a pilot embryo-fetal development study in rabbits, a dose of 30 mg/kg/day resulted in no viable fetuses (post-implantation loss). Doses ≥ 15 mg/kg/day resulted in increased embryo-fetal deaths, reduced fetal weights, and malformations, mostly related to the tail and head. At 15 mg/kg/day in rabbits, the maternal exposure was approximately 5 times the exposure achieved at the recommended human dose of 300 mg/day based on AUC.
8.2 Lactation
PIQRAY is used in combination with fulvestrant. Refer to the Full Prescribing Information of fulvestrant for lactation information.
There is no data on the presence of alpelisib in human milk, its effects on milk production, or the breastfed child. Because of the potential for serious adverse reactions in the breastfed child, advise lactating women to not breastfeed during treatment with PIQRAY and for 1 week after the last dose.
8.3 Females and Males of Reproductive Potential
PIQRAY is used in combination with fulvestrant. Refer to the Full Prescribing Information of fulvestrant for contraception and infertility information.
Pregnancy Testing
Verify the pregnancy status in females of reproductive potential prior to initiating PIQRAY.
Contraception
Females
PIQRAY can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)]. Advise females of reproductive potential to use effective contraception during treatment with PIQRAY and for 1 week after the last dose.
Males
Advise male patients with female partners of reproductive potential to use condoms and effective contraception during treatment with PIQRAY and for 1 week after the last dose.
Infertility
Based on findings from animal studies, PIQRAY may impair fertility in males and females of reproductive potential [see Nonclinical Toxicology (13.1)].
8.4 Pediatric Use
The safety and efficacy of PIQRAY in pediatric patients have not been established.
8.5 Geriatric Use
Of 284 patients who received PIQRAY in the SOLAR-1 trial, 117 patients were ≥ 65 years of age and 34 patients were ≥ 75 years of age. In patients treated with PIQRAY plus fulvestrant, there was a higher incidence of Grade 3-4 hyperglycemia in patients ≥ 65 years of age (44%) compared to patients < 65 years of age (32%). No overall differences in effectiveness of PIQRAY were observed between patients ≥ 65 years of age compared to younger patients. There are an insufficient number of patients ≥ 75 years of age to assess whether there are differences in safety or effectiveness. However, in the SOLAR-1 trial, an increase in the hyperglycemia adverse reactions (74% vs 66%) and Grade 3-4 (56% vs 36%) hyperglycemia were observed in patients ≥ 75 years of age compared to patients < 75 years of age, respectively [see Warnings and Precautions (5.3)].
10. Overdosage
There is limited experience of overdose with PIQRAY in clinical trials. In the clinical studies, PIQRAY was administered at doses up to 450 mg once daily.
In cases where accidental overdosage of PIQRAY was reported in the clinical studies, the adverse reactions associated with the overdose were consistent with the known safety profile of PIQRAY and included hyperglycemia, nausea, asthenia, and rash.
Initiate general symptomatic and supportive measures in all cases of overdosage where necessary. There is no known antidote for PIQRAY.
11. Piqray Description
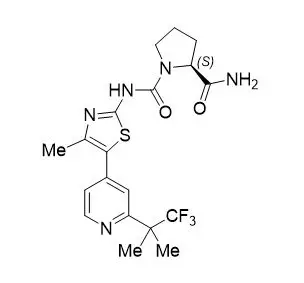
PIQRAY (alpelisib) is a kinase inhibitor. The chemical name of alpelisib is (2S)-N1-[4-Methyl-5-[2-(2,2,2-trifluoro-1,1-dimethylethyl)-4-pyridinyl]-2-thiazolyl]-1,2-pyrrolidinedicarboxamide. Alpelisib is a white to almost white powder. The molecular formula for alpelisib is C19H22F3N5O2S and the relative molecular mass is 441.47 g/mol. The chemical structure of alpelisib is shown below:
PIQRAY film-coated tablets are supplied for oral administration with three strengths that contain 50 mg, 150 mg and 200 mg of alpelisib. The tablets also contain hypromellose, magnesium stearate, mannitol, microcrystalline cellulose, and sodium starch glycolate. The film-coating contains hypromellose, iron oxide black, iron oxide red, macrogol/polyethylene glycol (PEG) 4000, talc, and titanium dioxide.
| PIQRAY
alpelisib tablet |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| PIQRAY
alpelisib kit |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| PIQRAY
alpelisib tablet |
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
|
||||||||||||||||||||||||
| Labeler - Novartis Pharmaceuticals Corporation (002147023) |