Drug Detail:Praxbind (Idarucizumab [ eye-da-roo-kiz-ue-mab ])
Drug Class: Anticoagulant reversal agents
Highlights of Prescribing Information
PRAXBIND® (idarucizumab) injection, for intravenous use
Initial U.S. Approval: 2015
Recent Major Changes
Indications and Usage for Praxbind
PRAXBIND is a humanized monoclonal antibody fragment (Fab) indicated in patients treated with Pradaxa® when reversal of the anticoagulant effects of dabigatran is needed:
- For emergency surgery/urgent procedures
- In life-threatening or uncontrolled bleeding (1)
Praxbind Dosage and Administration
For intravenous use only.
- The recommended dose of PRAXBIND is 5 g, provided as two separate vials each containing 2.5 g/50 mL idarucizumab. (2.1)
- There is limited data to support administration of an additional 5 g of PRAXBIND. (2.1)
Dosage Forms and Strengths
Injection: 2.5 g/50 mL solution in a single-dose vial (3)
Contraindications
- None (4)
Warnings and Precautions
- Thromboembolic Risk: Reversing dabigatran therapy exposes patients to the thrombotic risk of their underlying disease. Resume anticoagulant therapy as soon as medically appropriate. (2.4, 5.1)
- Re-elevation of Coagulation Parameters: In patients with elevated coagulation parameters and reappearance of clinically relevant bleeding or requiring a second emergency surgery/urgent procedure, an additional 5 g dose of PRAXBIND may be considered. (5.2)
- Hypersensitivity reactions: Discontinue administration and evaluate. (5.3)
- Risks of Serious Adverse Reactions in Patients with Hereditary Fructose Intolerance due to Sorbitol Excipient: Patients with hereditary fructose intolerance may be at risk of adverse reactions. (5.4)
Adverse Reactions/Side Effects
- In healthy volunteers, the most frequently reported adverse reactions in ≥5% of subjects treated with idarucizumab was headache. (6.1)
- In patients, the most frequently reported adverse reactions in ≥5% of patients treated with idarucizumab were constipation and nausea. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Boehringer Ingelheim Pharmaceuticals, Inc. at (800) 542-6257 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 10/2021
Related/similar drugs
idarucizumabFull Prescribing Information
Indications and Usage for Praxbind
- For emergency surgery/urgent procedures
- In life-threatening or uncontrolled bleeding
Praxbind Dosage and Administration
2.2 Preparation
- Remove both vials (each containing 2.5 g/50 mL idarucizumab) from carton.
- Ensure aseptic handling when preparing the infusion.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- Once solution has been removed from the vial, administration should begin promptly. The solution in vials may be stored at room temperature, 25°C (77°F), but must be used within 6 hours [see How Supplied/Storage and Handling (16.2)].
2.3 Administration
- Do not mix with other medicinal products. Use aseptic technique when administering PRAXBIND.
- Intravenously administer the dose of 5 g (2 vials, each
contains 2.5 g) as
- Two consecutive infusions (see Figure 2) or
- Bolus injection by injecting both vials consecutively one after another via syringe (see Figure 3).
- A pre-existing intravenous line may be used for administration of PRAXBIND. The line must be flushed with sterile 0.9% Sodium Chloride Injection, USP solution prior to infusion. No other infusion should be administered in parallel via the same intravenous access.
- PRAXBIND treatment can be used in conjunction with standard supportive measures, which should be considered as medically appropriate [see Clinical Pharmacology (12.2)].
 |  |  |
Warnings and Precautions
Adverse Reactions/Side Effects
The following serious adverse reactions are described in more detail elsewhere in the labeling:
- Thromboembolic Risk [see Warnings and Precautions (5.1)]
- Hypersensitivity Reactions [see Warnings and Precautions (5.3)]
- Risks of Serious Adverse Reactions in Patients with Hereditary Fructose Intolerance due to Sorbitol Excipient [see Warnings and Precautions (5.4)]
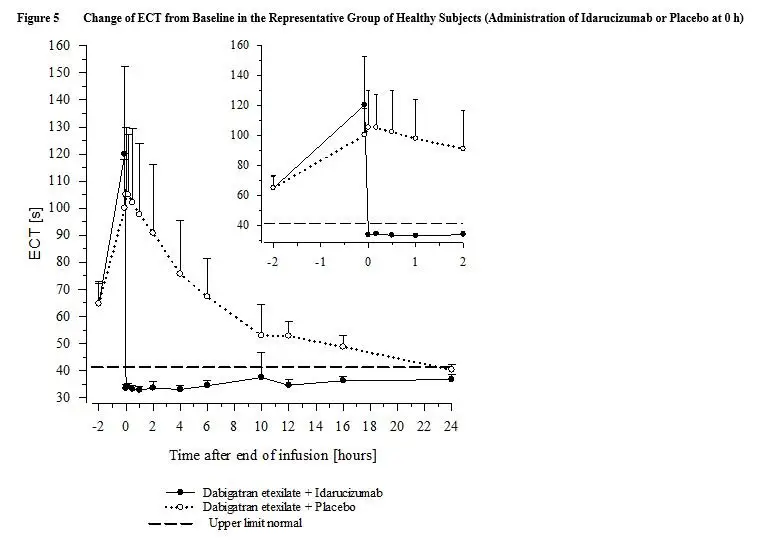
Praxbind - Clinical Pharmacology
Clinical Studies
| Clotting Assay (Mean and Standard Deviation) | Pre-Idarucizumab (N=14) | End of
infusion of Idarucizumab (N=14) | 24 hours
after Idarucizumab (N=14) |
| dTT [s] | 66.6 (12.0) | 32.1 (1.38) | 33.0 (1.69) |
| aPTT [s] | 67.8 (14.5) | 29.2 (4.74) | 31.9 (5.71) |
| ECT [s] | 122 (42.2) | 34.7 (1.92) | 38.8 (2.86) |
| TT [s] | 127 (62.6) | 12.5 (0.786) | 19.3 (5.14) |
| ACT [s] | 236 (47.6) | 116 (7.71) | 140 (10.1) |
| Clotting Assay (Mean and Standard Deviation) | Pre-Placebo (N=14) | End of
infusion of Placebo (N=14) | 24 hours
after Placebo (N=14) |
| dTT [s] | 64.7 (9.82) | 65.3 (12.1) | 36.1 (2.48) |
| aPTT [s] | 65.2 (14.0) | 66.5 (13.2) | 37.0 (7.10) |
| ECT [s] | 117 (29.8) | 122 (32.9) | 44.7 (5.39) |
| TT [s] | 132 (35.4) | 147 (46.7) | 39.5 (11.8) |
| ACT [s] | 219 (44.7) | 216 (50.5) | 148 (15.1) |
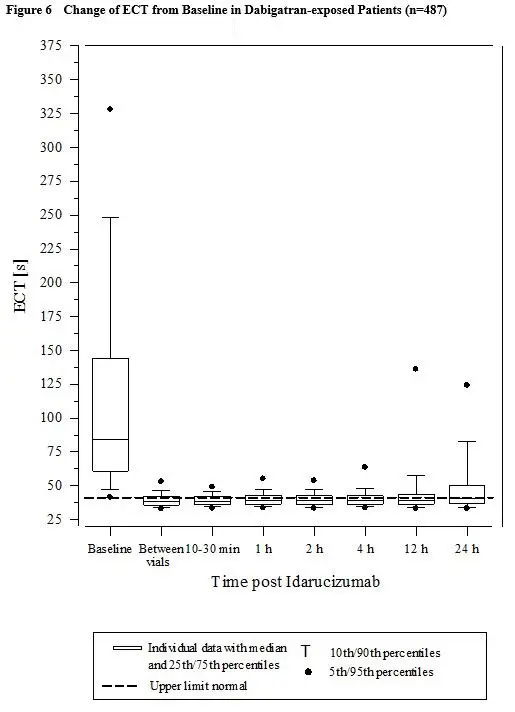
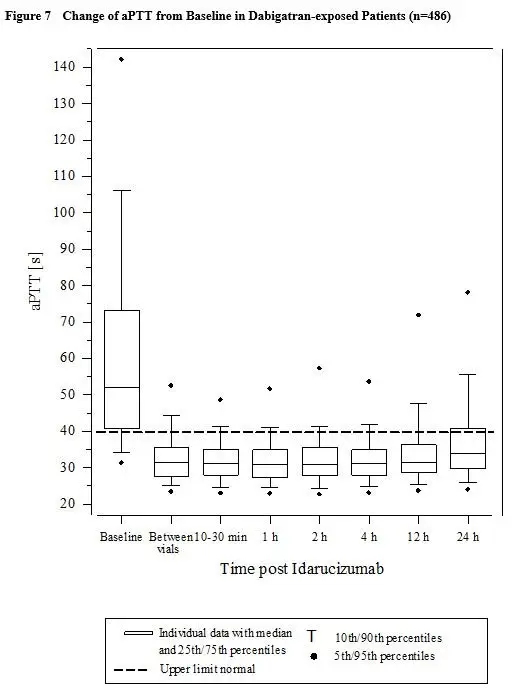
Activated partial thromboplastin time (aPTT) showed similar results to ECT (see Figure 7).
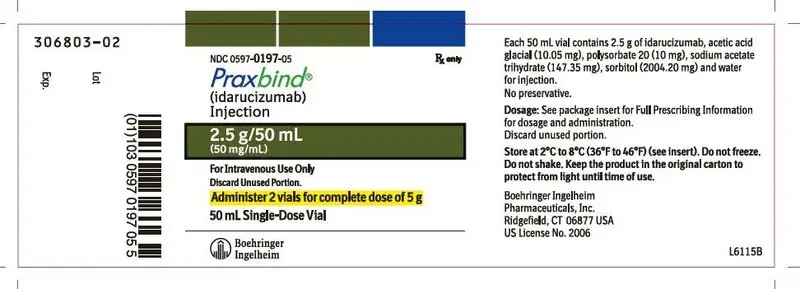
How is Praxbind supplied
16.1 How Supplied
- PRAXBIND is a sterile, preservative-free, colorless to slightly yellow, clear to slightly opalescent solution supplied as 2 single-dose vials each containing 2.5 g/50 mL of idarucizumab.
- NDC number 0597-0197-05: Carton containing two 2.5 g/50 mL vials.
16.2 Storage and Handling
- Store PRAXBIND vials in the refrigerator at 2ºC to 8ºC (36ºF to 46ºF) in the original carton to protect from light. Do not freeze. Do not shake.
- PRAXBIND vials may be stored at room temperature, 25°C (77°F), for up to 48 hours in the original carton to protect from light.
- PRAXBIND vials may be stored at room temperature, 25°C (77°F), out of the carton and exposed to light but must be used within 6 hours [see Dosage and Administration (2.2)].
| PRAXBIND
idarucizumab injection |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Registrant - Boehringer Ingelheim Pharmaceuticals, Inc. (603175944) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Boehringer Ingelheim Pharma GmbH and Co. KG | 340700520 | API MANUFACTURE(0597-0197) , MANUFACTURE(0597-0197) , PACK(0597-0197) , LABEL(0597-0197) , ANALYSIS(0597-0197) | |