Drug Detail:Primatene mist inhaler (Epinephrine inhalation [ ep-i-nef-rin ])
Drug Class: Adrenergic bronchodilators Catecholamines Vasopressors
DIRECTIONS FOR USE OF MOUTHPIECE
The Primatene Mist mouthpiece, which is enclosed in the Primatene Mist 15 mL size (not the refill size), should be used for inhalation only with Primatene Mist.
| 1. Take plastic cap off mouthpiece. (For refills, use mouthpiece from previous purchase.) | 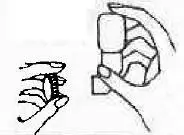 |
| 2. Take plastic mouthpiece off bottle. |  |
| 3. Place short end of mouthpiece on bottle. |  |
| 4. Turn bottle upside down. Place thumb on bottom of mouthpiece over circular button and forefinger on top of vial. Empty the lungs as completely as possible by exhaling. | 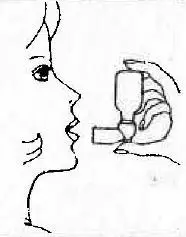 |
| 5. Place mouthpiece in mouth with lips closed around opening. Inhale deeply while squeezing mouthpiece and bottle together. Release immediately and remove unit from mouth, then complete taking the deep breath, drawing medication into your lungs, holding breath as long as comfortable. | 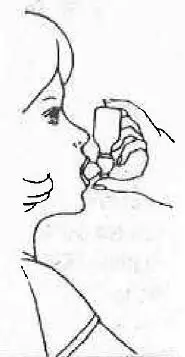 |
| 6. Exhale slowly keeping lips nearly closed. This helps distribute the medication in the lungs. | 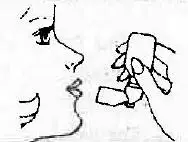 |
| 7. For storage, place long end of mouthpiece back on bottle and cover with plastic cap. | 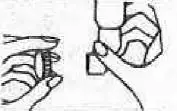 |
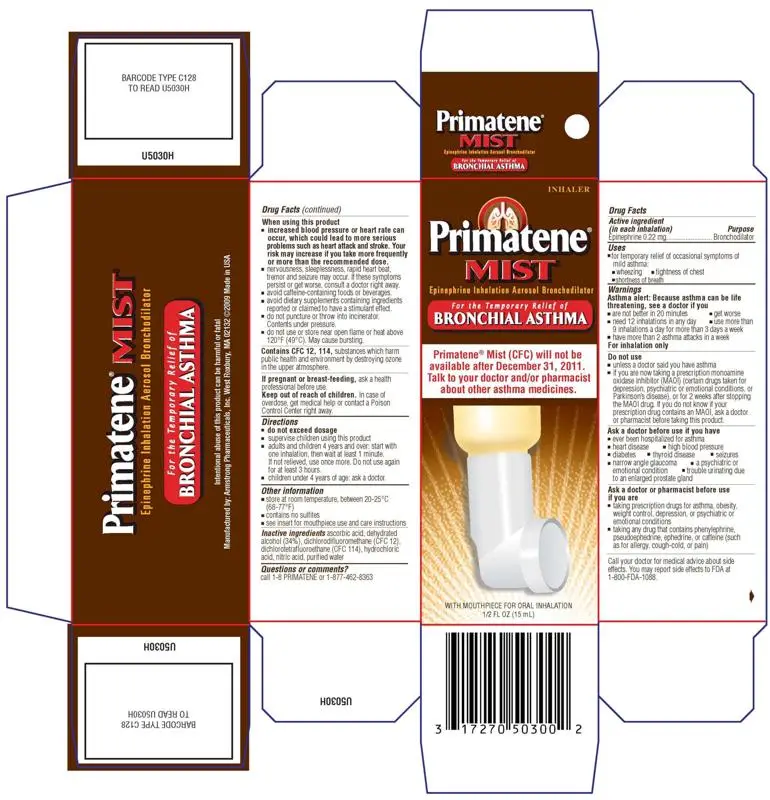
| PRIMATENE MIST
epinephrine inhalant |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
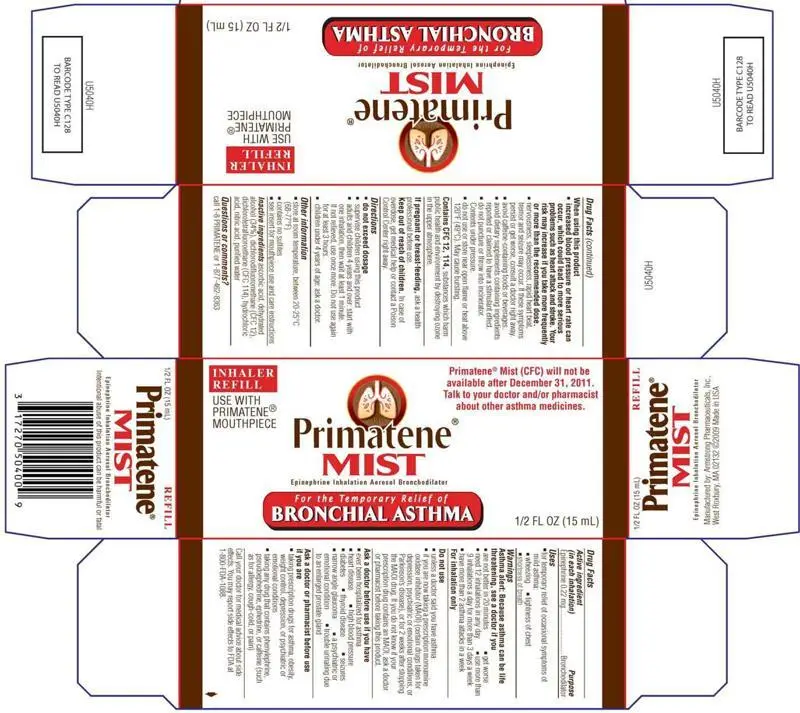
| PRIMATENE MIST REFILL
epinephrine inhalant |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Armstrong Pharmaceuticals, Inc. (001185115) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Armstrong Pharmaceuticals, Inc. | 001185115 | MANUFACTURE | |