Drug Detail:Prinivil (Lisinopril [ lyse-in-oh-pril ])
Drug Class: Angiotensin Converting Enzyme Inhibitors
Highlights of Prescribing Information
PRINIVIL® (lisinopril) tablets, for oral use
Initial U.S. Approval: 1987
WARNING: FETAL TOXICITY
See full prescribing information for complete boxed warning.
- When pregnancy is detected, discontinue PRINIVIL as soon as possible (5.1).
- Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus (5.1).
Indications and Usage for Prinivil
PRINIVIL is an angiotensin converting enzyme (ACE) inhibitor indicated for:
- Treatment of hypertension in adults and pediatric patients ≥6 years of age (1.1)
- Adjunctive therapy for heart failure (1.2)
- Treatment of acute myocardial infarction (1.3)
Prinivil Dosage and Administration
- Hypertension: Initiate adults at 10 mg (monotherapy) or 5 mg (on a diuretic) once daily. Titrate up to 40 mg daily based on response. Initial dose in patients 6 years of age and older is 0.07 mg/kg (up to 5 mg total) once daily (2.1)
- Heart Failure: Initiate with 5 mg once daily. Increase dose as tolerated to 40 mg daily (2.2)
- Acute Myocardial Infarction (MI): Give 5 mg within 24 hours of MI followed by 5 mg after 24 hours, then 10 mg once daily (2.3)
- Renal Impairment: For patients with creatinine clearance 10-30 mL/min, halve the usual initial dose. For creatinine clearance <10 mL/min or on hemodialysis, initiate at 2.5 mg (2.4)
Dosage Forms and Strengths
- Tablets (lisinopril content): 5 mg; 10 mg; and 20 mg (3)
Contraindications
- Angioedema or a history of hereditary or idiopathic angioedema (4)
- Hypersensitivity (4)
- Coadministration of aliskiren with PRINIVIL in patients with diabetes (4, 7.4)
- PRINIVIL is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer PRINIVIL within 36 hours of switching to or from sacubitril/valsartan, a product containing a neprilysin inhibitor (4)
Warnings and Precautions
- Angioedema: Discontinue PRINIVIL (5.2)
- Renal impairment: Monitor renal function periodically (5.3)
- Hypotension: Monitor blood pressure after initiation (5.4)
- Hyperkalemia: Monitor serum potassium periodically (5.5)
- Cholestatic jaundice and hepatic failure: Discontinue PRINIVIL (5.6)
Adverse Reactions/Side Effects
Common adverse reactions (events 2% greater than on placebo):
- Hypertension: headache, dizziness and cough (6.1)
- Heart Failure: hypotension and chest pain (6.1)
- Acute Myocardial Infarction: hypotension (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., at 1-877-888-4231 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch .
Drug Interactions
- Diuretics: Excessive drop in blood pressure (7.1)
- NSAIDs: Increased risk of renal impairment and loss of antihypertensive efficacy (7.3)
- Dual inhibition of the renin-angiotensin system: Increased risk of renal impairment, hypotension, syncope, and hyperkalemia (7.4)
- Lithium: Symptoms of lithium toxicity (7.5)
- Gold: Nitritoid reactions (7.6)
Use In Specific Populations
- Pregnancy: Discontinue PRINIVIL if pregnancy is detected (5.1, 8.1)
- Pediatrics: Safety and effectiveness have not been established in patients <6 years of age or with glomerular filtration rate <30 mL/min/1.73 m2 (8.4)
- Race: Less antihypertensive effect in Blacks than non-Blacks (8.6)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2021
Full Prescribing Information
WARNING: FETAL TOXICITY
When pregnancy is detected, discontinue PRINIVIL as soon as possible [see Warnings and Precautions (5.1)].
Drugs that act directly on the renin-angiotensin system can cause injury and death to the developing fetus [see Warnings and Precautions (5.1)].
1. Indications and Usage for Prinivil
1.1 Hypertension
PRINIVIL is indicated for the treatment of hypertension in adult patients and pediatric patients 6 years of age and older to lower blood pressure. Lowering blood pressure lowers the risk of fatal and non-fatal cardiovascular events, primarily strokes and myocardial infarctions. These benefits have been seen in controlled trials of antihypertensive drugs from a wide variety of pharmacologic classes.
Control of high blood pressure should be part of comprehensive cardiovascular risk management, including, as appropriate, lipid control, diabetes management, antithrombotic therapy, smoking cessation, exercise, and limited sodium intake. Many patients will require more than 1 drug to achieve blood pressure goals. For specific advice on goals and management, see published guidelines, such as those of the National High Blood Pressure Education Program's Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure (JNC).
Numerous antihypertensive drugs, from a variety of pharmacologic classes and with different mechanisms of action, have been shown in randomized controlled trials to reduce cardiovascular morbidity and mortality, and it can be concluded that it is blood pressure reduction, and not some other pharmacologic property of the drugs, that is largely responsible for those benefits. The largest and most consistent cardiovascular outcome benefit has been a reduction in the risk of stroke, but reductions in myocardial infarction and cardiovascular mortality also have been seen regularly.
Elevated systolic or diastolic pressure causes increased cardiovascular risk, and the absolute risk increase per mmHg is greater at higher blood pressures, so that even modest reductions of severe hypertension can provide substantial benefit. Relative risk reduction from blood pressure reduction is similar across populations with varying absolute risk, so the absolute benefit is greater in patients who are at higher risk independent of their hypertension (for example, patients with diabetes or hyperlipidemia), and such patients would be expected to benefit from more aggressive treatment to a lower blood pressure goal.
Some antihypertensive drugs have smaller blood pressure effects (as monotherapy) in Black patients, and many antihypertensive drugs have additional approved indications and effects (e.g., on angina, heart failure, or diabetic kidney disease). These considerations may guide selection of therapy.
PRINIVIL may be administered alone or with other antihypertensive agents [see Clinical Studies (14.1)].
1.2 Heart Failure
PRINIVIL is indicated to reduce signs and symptoms of heart failure in patients who are not responding adequately to diuretics and digitalis [see Clinical Studies (14.2)].
1.3 Acute Myocardial Infarction
PRINIVIL is indicated for the reduction of mortality in treatment of hemodynamically stable patients within 24 hours of acute myocardial infarction. Patients should receive, as appropriate, the standard recommended treatments such as thrombolytics, aspirin and beta-blockers [see Clinical Studies (14.3)].
2. Prinivil Dosage and Administration
2.1 Hypertension
Initial therapy in adults: The recommended initial dose is 10 mg once a day. Adjust dosage according to blood pressure response. The usual dosage range is 20 to 40 mg per day administered in a single daily dose. Doses up to 80 mg have been used but do not appear to give a greater effect.
2.2 Heart Failure
The recommended starting dose for PRINIVIL, when used with diuretics and (usually) digitalis as adjunctive therapy is 5 mg once daily. The recommended starting dose in these patients with hyponatremia (serum sodium <130 mEq/L) is 2.5 mg once daily. Increase as tolerated to a maximum of 40 mg once daily.
Diuretic dose may need to be adjusted to help minimize hypovolemia, which may contribute to hypotension [see Warnings and Precautions (5.4) and Drug Interactions (7.1)]. The appearance of hypotension after the initial dose of PRINIVIL does not preclude subsequent careful dose titration with the drug, following effective management of the hypotension.
2.3 Acute Myocardial Infarction
In hemodynamically stable patients within 24 hours of the onset of symptoms of acute myocardial infarction, give PRINIVIL 5 mg orally, followed by 5 mg after 24 hours, 10 mg after 48 hours and then 10 mg once daily. Dosing should continue for at least 6 weeks.
Initiate therapy with 2.5 mg in patients with a low systolic blood pressure (100-120 mmHg) during the first 3 days after the infarct [see Warnings and Precautions (5.4)]. If hypotension occurs (systolic blood pressure ≤100 mmHg) consider doses of 2.5 or 5 mg. If prolonged hypotension occurs (systolic blood pressure <90 mmHg for more than 1 hour) discontinue PRINIVIL.
2.4 Dose in Patients with Renal Impairment
No dose adjustment of PRINIVIL is required in patients with creatinine clearance >30 mL/min. In patients with creatinine clearance 10-30 mL/min, reduce the initial dose of PRINIVIL to half of the usual recommended dose (i.e., hypertension, 5 mg; heart failure or acute MI, 2.5 mg). For patients on hemodialysis or creatinine clearance <10 mL/min, the recommended initial dose is 2.5 mg once daily [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.5 Preparation of Suspension
To make 200 mL of a suspension at 1.0 mg/mL, add 10 mL of Purified Water USP to a polyethylene terephthalate (PET) bottle containing ten 20-mg tablets of PRINIVIL and shake for at least one minute. Add 30 mL of Sodium Citrate and Citric Acid Oral Solution or Cytra-2 diluent and 160 mL of Ora-Sweet SF™ to the concentrate in the PET bottle and gently shake for several seconds to disperse the ingredients. The suspension should be stored at or below 25°C (77°F) and can be stored for up to four weeks. Shake the suspension before each use.
3. Dosage Forms and Strengths
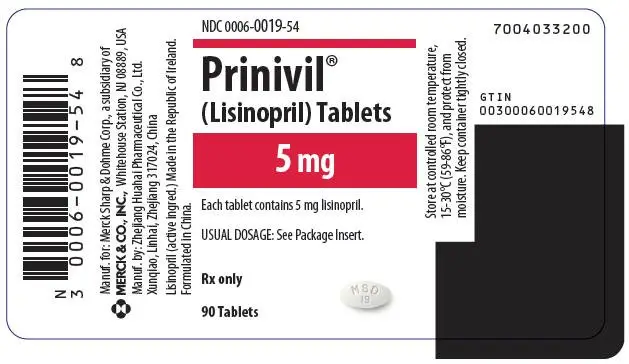
Tablets PRINIVIL, 5 mg, are white, oval-shaped compressed tablets with code MSD 19 on one side and scored on the other side.
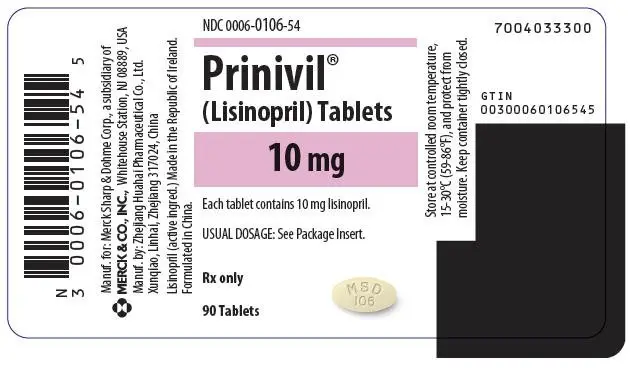
Tablets PRINIVIL, 10 mg, are light yellow, oval-shaped compressed tablets with code MSD 106 on one side and scored on the other side.
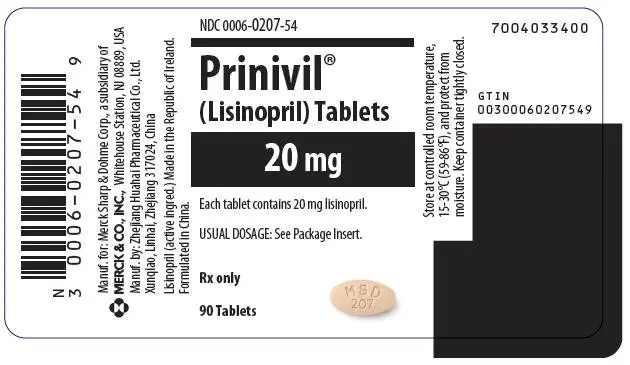
Tablets PRINIVIL, 20 mg, are peach, oval-shaped compressed tablets with code MSD 207 on one side and scored on the other side.
4. Contraindications
PRINIVIL is contraindicated in patients with:
- a history of angioedema or hypersensitivity related to previous treatment with an angiotensin converting enzyme inhibitor
- hereditary or idiopathic angioedema.
Do not coadminister aliskiren with PRINIVIL in patients with diabetes [see Drug Interactions (7.4)].
PRINIVIL is contraindicated in combination with a neprilysin inhibitor (e.g., sacubitril). Do not administer PRINIVIL within 36 hours of switching to or from sacubitril/valsartan, a product containing a neprilysin inhibitor [see Warnings and Precautions (5.2) and Drug Interactions (7.8)].
5. Warnings and Precautions
5.3 Impaired Renal Function
Monitor renal function periodically in patients treated with PRINIVIL. Changes in renal function including acute renal failure can be caused by drugs that inhibit the renin-angiotensin system. Patients whose renal function may depend in part on the activity of the renin-angiotensin system (e.g., patients with renal artery stenosis, chronic kidney disease, severe congestive heart failure, post-myocardial infarction or volume depletion) may be at particular risk of developing acute renal failure on PRINIVIL. Consider withholding or discontinuing therapy in patients who develop a clinically significant decrease in renal function on PRINIVIL [see Adverse Reactions (6.1) and Drug Interactions (7.4)].
5.4 Hypotension
PRINIVIL can cause symptomatic hypotension, sometimes complicated by oliguria, progressive azotemia, acute renal failure or death. Patients at risk of excessive hypotension include those with the following conditions or characteristics: heart failure with systolic blood pressure below 100 mmHg, ischemic heart disease, cerebrovascular disease, hyponatremia, high dose diuretic therapy, renal dialysis, or severe volume and/or salt depletion of any etiology.
In these patients, start PRINIVIL under medical supervision and follow such patients for the first two weeks of treatment and whenever the dose of PRINIVIL and/or diuretic is increased. Avoid use of PRINIVIL in patients who are hemodynamically unstable after acute MI.
Symptomatic hypotension is also possible in patients with severe aortic stenosis or hypertrophic cardiomyopathy.
5.5 Hyperkalemia
Monitor serum potassium periodically in patients receiving PRINIVIL. Drugs that inhibit the renin-angiotensin system can cause hyperkalemia. Risk factors for the development of hyperkalemia include renal insufficiency, diabetes mellitus, and the concomitant use of potassium-sparing diuretics, potassium supplements, potassium-containing salt substitutes, or other drugs that may increase serum potassium [see Drug Interactions (7.1)].
5.6 Hepatic Failure
ACE inhibitors have been associated with a syndrome that starts with cholestatic jaundice or hepatitis and progresses to fulminant hepatic necrosis and sometimes death. The mechanism of this syndrome is not understood. Patients receiving ACE inhibitors who develop jaundice or marked elevations of hepatic enzymes should discontinue the ACE inhibitor and receive appropriate medical treatment.
6. Adverse Reactions/Side Effects
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of lisinopril that are not included in other sections of labeling. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Other reactions include:
Metabolism and nutrition disorders
Hyponatremia [see Warnings and Precautions (5.4)], cases of hypoglycemia in diabetic patients on oral antidiabetic agents or insulin [see Drug Interactions (7.2)]
Nervous system and psychiatric disorders
Mood alterations (including depressive symptoms), mental confusion
7. Drug Interactions
7.1 Diuretics
Initiation of PRINIVIL in patients on diuretics may result in excessive reduction of blood pressure. The possibility of hypotensive effects with PRINIVIL can be minimized by either decreasing or discontinuing the diuretic or increasing the salt intake prior to initiation of treatment with PRINIVIL. If this is not possible, reduce the starting dose of PRINIVIL [see Dosage and Administration (2.2) and Warnings and Precautions (5.4)].
PRINIVIL attenuates potassium loss caused by thiazide-type diuretics. Potassium-sparing diuretics (spironolactone, amiloride, triamterene, and others) or other drugs that may increase serum potassium can increase the risk of hyperkalemia. Therefore, if concomitant use of such agents is indicated, monitor the patient's serum potassium frequently.
7.2 Antidiabetics
Concomitant administration of PRINIVIL and antidiabetic medicines (insulins, oral hypoglycemic agents) may cause an increased blood-glucose-lowering effect with risk of hypoglycemia.
7.3 Non-Steroidal Anti-Inflammatory Agents Including Selective Cyclooxygenase-2 Inhibitors (COX-2 Inhibitors)
In patients who are elderly, volume-depleted (including those on diuretic therapy), or with compromised renal function, coadministration of NSAIDs, including selective COX-2 inhibitors, with ACE inhibitors, including lisinopril, may result in deterioration of renal function, including possible acute renal failure. These effects are usually reversible. Monitor renal function periodically in patients receiving lisinopril and NSAID therapy.
The antihypertensive effect of ACE inhibitors, including lisinopril, may be attenuated by NSAIDs.
7.4 Dual Blockade of the Renin-Angiotensin System (RAS)
Dual blockade of the RAS with angiotensin receptor blockers, ACE inhibitors, or direct renin inhibitors (such as aliskiren) is associated with increased risks of hypotension, syncope, hyperkalemia, and changes in renal function (including acute renal failure) compared to monotherapy.
The Veterans Affairs Nephropathy in Diabetes (VA NEPHRON-D) trial enrolled 1448 patients with type 2 diabetes, elevated urinary-albumin-to-creatinine ratio, and decreased estimated glomerular filtration rate (GFR 30 to 89.9 ml/min), randomized them to lisinopril or placebo on a background of losartan therapy and followed them for a median of 2.2 years. Patients receiving the combination of losartan and lisinopril did not obtain any additional benefit compared to monotherapy for the combined endpoint of decline in GFR, end stage renal disease, or death, but experienced an increased incidence of hyperkalemia and acute kidney injury compared with the monotherapy group.
In general, avoid combined use of RAS inhibitors. Monitor blood pressure, renal function and electrolytes in patients on PRINIVIL and other agents that affect the RAS.
Do not coadminister aliskiren with PRINIVIL in patients with diabetes. Avoid use of aliskiren with PRINIVIL in patients with renal impairment (GFR <60 ml/min).
7.5 Lithium
Lithium toxicity has been reported in patients receiving lithium concomitantly with drugs, which cause elimination of sodium, including ACE inhibitors. Lithium toxicity was usually reversible upon discontinuation of lithium and the ACE inhibitor. Monitor serum lithium levels during concurrent use.
7.6 Gold
Nitritoid reactions (symptoms include facial flushing, nausea, vomiting and hypotension) have been reported rarely in patients on therapy with injectable gold (sodium aurothiomalate) and concomitant ACE inhibitor therapy including PRINIVIL.
8. Use In Specific Populations
8.2 Lactation
Risk Summary
No data are available regarding the presence of lisinopril in human milk or the effects of lisinopril on the breastfed infant or on milk production. Lisinopril is present in rat milk. Because many drugs are secreted in human milk, and because of the potential for serious adverse reactions in the breastfed infants from ACE inhibitors, discontinue breastfeeding or discontinue PRINIVIL.
8.4 Pediatric Use
Antihypertensive effects and safety of PRINIVIL have been established in pediatric patients aged 6 to 16 years [see Dosage and Administration (2.1) and Clinical Studies (14.1)]. No relevant differences between the adverse reaction profile for pediatric patients and adult patients were identified.
Safety and effectiveness of PRINIVIL have not been established in pediatric patients under the age of 6 or in pediatric patients with glomerular filtration rate <30 mL/min/1.73 m2 [see Clinical Pharmacology (12.3) and Clinical Studies (14.1)].
8.5 Geriatric Use
No dosage adjustment with PRINIVIL is necessary in elderly patients. In a clinical study of PRINIVIL in patients with myocardial infarctions (GISSI-3 Trial) 4,413 (47%) were 65 and over, while 1,656 (18%) were 75 and over. In this study, 4.8% of patients aged 75 years and older discontinued PRINIVIL treatment because of renal dysfunction vs. 1.3% of patients younger than 75 years. No other differences in safety or effectiveness were observed between elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
8.6 Race
ACE inhibitors, including PRINIVIL, have an effect on blood pressure that is less in Black patients than in non-Blacks.
8.7 Renal Impairment
Dose adjustment of PRINIVIL is required in patients undergoing hemodialysis or whose creatinine clearance is ≤30 mL/min. No dose adjustment of PRINIVIL is required in patients with creatinine clearance >30 mL/min [see Dosage and Administration (2.4) and Clinical Pharmacology (12.3)].
10. Overdosage
Following a single oral dose of 20 g/kg, no lethality occurred in rats and death occurred in one of 20 mice receiving the same dose. The most likely manifestation of overdosage would be hypotension, for which the usual treatment would be intravenous infusion of normal saline solution.
Lisinopril can be removed by hemodialysis [see Warnings and Precautions (5.2)].
11. Prinivil Description
PRINIVIL contains lisinopril, a synthetic peptide derivative, and an oral, long-acting angiotensin converting enzyme inhibitor. Lisinopril is chemically described as (S)-1-[N2-(1-carboxy-3-phenylpropyl)-L-lysyl]-L-proline dihydrate. Its empirical formula is C21H31N3O5∙2H2O and its structural formula is:
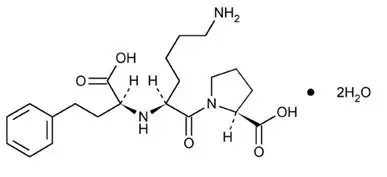 |
Lisinopril is a white to off-white, crystalline powder, with a molecular weight of 441.52. It is soluble in water and sparingly soluble in methanol and practically insoluble in ethanol.
PRINIVIL is supplied as 5 mg, 10 mg, and 20 mg tablets for oral administration. In addition to the active ingredient, lisinopril, each tablet contains the following inactive ingredients: calcium phosphate, mannitol, magnesium stearate, and starch. The 10 mg and 20 mg tablets also contain iron oxide.
12. Prinivil - Clinical Pharmacology
12.1 Mechanism of Action
Lisinopril inhibits angiotensin converting enzyme (ACE) in human subjects and animals. ACE is a peptidyl dipeptidase that catalyzes the conversion of angiotensin I to the vasoconstrictor substance, angiotensin II. Angiotensin II also stimulates aldosterone secretion by the adrenal cortex. The beneficial effects of lisinopril in hypertension and heart failure appear to result primarily from suppression of the renin-angiotensin-aldosterone system. Inhibition of ACE results in decreased plasma angiotensin II which leads to decreased vasopressor activity and to decreased aldosterone secretion. The latter decrease may result in a small increase of serum potassium. In hypertensive patients with normal renal function treated with PRINIVIL alone for up to 24 weeks, the mean increase in serum potassium was approximately 0.1 mEq/L; however, approximately 15% of patients had increases greater than 0.5 mEq/L and approximately 6% had a decrease greater than 0.5 mEq/L. In the same study, patients treated with PRINIVIL and hydrochlorothiazide for up to 24 weeks had a mean decrease in serum potassium of 0.1 mEq/L; approximately 4% of patients had increases greater than 0.5 mEq/L and approximately 12% had a decrease greater than 0.5 mEq/L [see Warnings and Precautions (5.5)]. Removal of angiotensin II negative feedback on renin secretion leads to increased plasma renin activity.
ACE is identical to kininase, an enzyme that degrades bradykinin. Whether increased levels of bradykinin, a potent vasodepressor peptide, play a role in the therapeutic effects of PRINIVIL remains to be elucidated.
While the mechanism through which PRINIVIL lowers blood pressure is believed to be primarily suppression of the renin-angiotensin-aldosterone system, PRINIVIL is antihypertensive even in patients with low-renin hypertension. Although PRINIVIL was antihypertensive in all races studied, Black hypertensive patients (usually a low-renin hypertensive population) had a smaller average response to monotherapy than non-Black patients.
Concomitant administration of PRINIVIL and hydrochlorothiazide further reduced blood pressure in Black and non-Black patients and any racial difference in blood pressure response was no longer evident.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
There was no evidence of a tumorigenic effect when lisinopril was administered for 105 weeks to male and female rats at doses up to 90 mg per kg per day or for 92 weeks to male and female mice at doses up to 135 mg per kg per day. These doses are 10 times and 7 times, respectively, the MRHDD when compared on a body surface area basis.
Lisinopril was not mutagenic in the Ames microbial mutagen test with or without metabolic activation. It was also negative in a forward mutation assay using Chinese hamster lung cells. Lisinopril did not produce single strand DNA breaks in an in vitro alkaline elution rat hepatocyte assay. In addition, lisinopril did not produce increases in chromosomal aberrations in an in vitro test in Chinese hamster ovary cells or in an in vivo study in mouse bone marrow.
There were no adverse effects on reproductive performance in male and female rats treated with up to 300 mg/kg/day of lisinopril (33 times the MRHDD when compared on a body surface area basis).
Studies in rats indicate that lisinopril crosses the blood brain barrier poorly. Multiple doses of lisinopril in rats do not result in accumulation in any tissues. Milk of lactating rats contains radioactivity following administration of 14C lisinopril. By whole body autoradiography, radioactivity was found in the placenta following administration of labeled drug to pregnant rats, but none was found in the fetuses.
14. Clinical Studies
14.2 Heart Failure
In two placebo controlled, 12-week clinical studies compared the addition of PRINIVIL up to 20 mg daily to digitalis and diuretics alone. The combination of PRINIVIL, digitalis and diuretics reduced the following signs and symptoms of heart failure: edema, rales, paroxysmal nocturnal dyspnea and jugular venous distention. In one of the studies, the combination of PRINIVIL, digitalis and diuretics reduced orthopnea, presence of third heart sound and the number of patients classified as NYHA Class III and IV, and it improved exercise tolerance. A large (over 3000 patients) survival study, the ATLAS Trial, comparing 2.5 and 35 mg of lisinopril in patients with systolic heart failure, showed that the higher dose of lisinopril had outcomes at least as favorable as the lower dose. During baseline-controlled clinical trials, in patients receiving digitalis and diuretics, single doses of PRINIVIL resulted in decreases in pulmonary capillary wedge pressure, systemic vascular resistance and blood pressure accompanied by an increase in cardiac output and no change in heart rate.
14.3 Acute Myocardial Infarction
The Gruppo Italiano per lo Studio della Sopravvienza nell'Infarto Miocardico (GISSI-3) study was a multicenter, controlled, randomized, unblinded clinical trial conducted in 19,394 patients with acute myocardial infarction (MI) admitted to a coronary care unit. It was designed to examine the effects of short-term (6 week) treatment with lisinopril, nitrates, their combination, or no therapy on short-term (6 week) mortality and on long-term death and markedly impaired cardiac function. Hemodynamically-stable patients presenting within 24 hours of the onset of symptoms were randomized, in a 2 × 2 factorial design, to 6 weeks of either 1) PRINIVIL alone (n=4841), 2) nitrates alone (n=4869), 3) PRINIVIL plus nitrates (n=4841), or 4) open control (n=4843). All patients received routine therapies, including thrombolytics (72%), aspirin (84%), and a beta blocker (31%), as appropriate, normally utilized in acute myocardial infarction (MI) patients.
The protocol excluded patients with hypotension (systolic blood pressure ≤100 mmHg), severe heart failure, cardiogenic shock, and renal dysfunction (serum creatinine >2 mg/dL and/or proteinuria >500 mg per 24 h). Patients randomized to PRINIVIL received 5 mg within 24 hours of the onset of symptoms, 5 mg after 24 hours, and then 10 mg daily thereafter. Patients with systolic blood pressure less than 120 mmHg at baseline received 2.5 mg of PRINIVIL. If hypotension occurred, the PRINIVIL dose was reduced or if severe hypotension occurred PRINIVIL was stopped [see Dosage and Administration (2.3)].
The primary outcomes of the trial were the overall mortality at 6 weeks and a combined endpoint at 6 months after the myocardial infarction, consisting of the number of patients who died, had late (day 4) clinical congestive heart failure, or had extensive left ventricular damage defined as ejection fraction ≤35%, or an akinetic-dyskinetic [A-D] score ≥45%. Patients receiving PRINIVIL (n=9646), alone or with nitrates, had an 11% lower risk of death (p =0.04) compared to patients who did not receive PRINIVIL (n=9672) (6.4% vs. 7.2%, respectively) at 6 weeks. Although patients randomized to receive PRINIVIL for up to 6 weeks also fared numerically better on the combined endpoint at 6 months, the open nature of the assessment of heart failure, substantial loss to follow-up echocardiography, and substantial excess use of PRINIVIL, between 6 weeks and 6 months in the group randomized to 6 weeks of lisinopril, preclude any conclusion about this endpoint.
Patients with acute myocardial infarction, treated with PRINIVIL, had a higher (9.0% versus 3.7%) incidence of persistent hypotension (systolic blood pressure <90 mmHg for more than 1 hour) and renal dysfunction (2.4% versus 1.1%) in-hospital and at 6 weeks (increasing creatinine concentration to over 3 mg/dL or a doubling or more of the baseline serum creatinine concentration) [see Adverse Reactions (6.1)].
16. How is Prinivil supplied
PRINIVIL is supplied as oval-shaped, compressed tablets scored on one side.
| Color | Printing | Unit of use Bottle/90 |
|
|---|---|---|---|
| 5 mg | White | MSD 19 | NDC 0006-0019-54 |
| 10 mg | Light yellow | MSD 106 | NDC 0006-0106-54 |
| 20 mg | Peach | MSD 207 | NDC 0006-0207-54 |
| PRINIVIL
lisinopril tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| PRINIVIL
lisinopril tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| PRINIVIL
lisinopril tablet |
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
|
||||||||||||||||||
| Labeler - Merck Sharp & Dohme Corp. (001317601) |