Drug Detail:Pristiq (Desvenlafaxine [ des-ven-la-fax-een ])
Drug Class: Serotonin-norepinephrine reuptake inhibitors
Highlights of Prescribing Information
PRISTIQ® (desvenlafaxine) Extended-Release Tablets, for oral use
Initial U.S. Approval: 2008
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning.
- Increased the risk of suicidal thoughts and behaviors in children, adolescents and young adults taking antidepressants (5.1).
- Closely monitor for clinical worsening and emergence of suicidal thoughts and behaviors (5.1).
- PRISTIQ is not approved for use in pediatric patients (8.4).
Recent Major Changes
| Dosage and Administration, Discontinuing PRISTIQ (2.5) | 11/2021 |
| Warnings and Precautions, Discontinuation Syndrome (5.7) | 11/2021 |
| Warnings and Precautions, Sexual Dysfunction (5.11) | 9/2021 |
Indications and Usage for Pristiq
PRISTIQ is a serotonin and norepinephrine reuptake inhibitor (SNRI) indicated for the treatment of adults with major depressive disorder (MDD) (1).
Pristiq Dosage and Administration
- Recommended dose: 50 mg once daily with or without food (2.1).
- There was no evidence that doses greater than 50 mg per day confer any additional benefit (2.1).
- The 25 mg per day dose is intended for a gradual reduction in dose when discontinuing treatment or dosing in severe renal and end-stage renal disease patients (2.1).
- Discontinuation: Reduce dose gradually whenever possible (2.1).
- Take tablets whole; do not divide, crush, chew, or dissolve (2.1).
- Moderate renal impairment: Maximum dose 50 mg per day (2.2).
- Severe renal impairment and end-stage renal disease: Maximum dose 25 mg per day or 50 mg every other day (2.2).
- Moderate to severe hepatic impairment: Maximum dose 100 mg per day (2.3).
Dosage Forms and Strengths
- PRISTIQ extended-release tablets: 25 mg, 50 mg and 100 mg (3).
- Each tablet contains 38 mg, 76 mg or 152 mg of desvenlafaxine succinate equivalent to 25 mg, 50 mg or 100 mg of desvenlafaxine, respectively (3).
Contraindications
- Hypersensitivity to desvenlafaxine succinate, venlafaxine hydrochloride or any excipients in the PRISTIQ formulation (4).
- Serotonin Syndrome and MAOIs: Do not use MAOIs intended to treat psychiatric disorders with PRISTIQ or within 7 days of stopping treatment with PRISTIQ. Do not use PRISTIQ within 14 days of stopping an MAOI intended to treat psychiatric disorders. In addition, do not start PRISTIQ in a patient who is being treated with linezolid or intravenous methylene blue (4).
Warnings and Precautions
- Serotonin Syndrome: Increased risk when co-administered with other serotonergic agents (e.g., SSRI, SNRI, triptans), but also when taken alone. If it occurs, discontinue PRISTIQ and initiate supportive treatment (5.2).
- Elevated Blood Pressure: Control hypertension before initiating treatment. Monitor blood pressure regularly during treatment (5.3).
- Increased Risk of Bleeding: Concomitant use of aspirin, NSAIDs, other antiplatelet drugs, warfarin, and other anticoagulants may increase this risk (5.4).
- Angle Closure Glaucoma: Avoid use of antidepressants, including PRISTIQ, in patients with untreated anatomically narrow angles treated (5.5).
- Activation of Mania/Hypomania: Use cautiously in patients with Bipolar Disorder. Caution patients about risk of activation of mania/hypomania (5.6).
- Discontinuation Syndrome: Taper dose when possible and monitor for discontinuation symptoms (5.7).
- Seizure: Can occur. Use cautiously in patients with seizure disorder (5.8).
- Hyponatremia: Can occur in association with SIADH (5.9).
- Interstitial Lung Disease and Eosinophilic Pneumonia: Can occur (5.10).
- Sexual Dysfunction: PRISTIQ may cause symptoms of sexual dysfunction (5.11).
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥5% and twice the rate of placebo in the 50 or 100 mg dose groups) were: nausea, dizziness, insomnia, hyperhidrosis, constipation, somnolence, decreased appetite, anxiety, and specific male sexual function disorders (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc., at 1-800-438-1985 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
- Pregnancy: Third trimester use may result in neonatal discontinuation syndrome (8.1).
- Geriatric Use: There is an increased incidence of orthostatic hypotension in desvenlafaxine treated patients ≥ 65 years (6.1 and 8.5).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 11/2021
Related/similar drugs
sertraline, trazodone, Lexapro, citalopram, Zoloft, Cymbalta, ProzacFull Prescribing Information
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
Antidepressants increased the risk of suicidal thoughts and behavior in children, adolescents, and young adults in short-term studies. These studies did not show an increase in the risk of suicidal thoughts and behavior with antidepressant use in patients over age 24; there was a reduction in risk with antidepressant use in patients aged 65 and older [see Warnings and Precautions (5.1)].
In patients of all ages who are started on antidepressant therapy, monitor closely for worsening, and for emergence of suicidal thoughts and behaviors. Advise families and caregivers of the need for close observation and communication with the prescriber [see Warnings and Precautions (5.1)].
PRISTIQ is not approved for use in pediatric patients [see Use in Specific Populations (8.4)].
1. Indications and Usage for Pristiq
PRISTIQ is indicated for the treatment of adults with major depressive disorder (MDD) [see Clinical Studies (14)].
2. Pristiq Dosage and Administration
2.1 General Instructions for Use
The recommended dose for PRISTIQ is 50 mg once daily, with or without food. The 50 mg dose is both a starting dose and the therapeutic dose. PRISTIQ should be taken at approximately the same time each day. Tablets must be swallowed whole with fluid and not divided, crushed, chewed, or dissolved.
In clinical studies, doses of 10 mg to 400 mg per day were studied. In clinical studies, doses of 50 mg to 400 mg per day were shown to be effective, although no additional benefit was demonstrated at doses greater than 50 mg per day and adverse reactions and discontinuations were more frequent at higher doses.
The 25 mg per day dose is intended for a gradual reduction in dose when discontinuing treatment. When discontinuing therapy, gradual dose reduction is recommended whenever possible to minimize discontinuation symptoms [see Dosage and Administration (2.5) and Warnings and Precautions (5.7)].
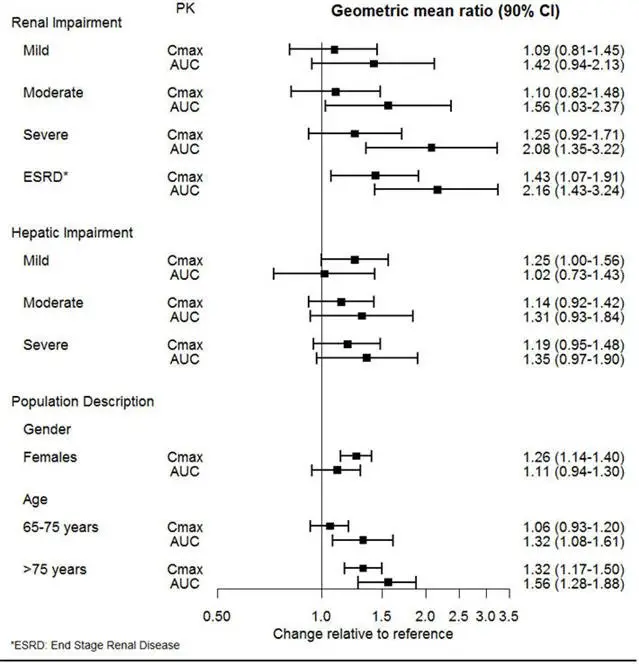
2.2 Dosage Recommendations for Patients with Renal Impairment
The maximum recommended dose in patients with moderate renal impairment (24-hr creatinine clearance [ClCr] = 30 to 50 mL/min, Cockcroft-Gault [C-G]) is 50 mg per day. The maximum recommended dose in patients with severe renal impairment (ClCr 15 to 29 mL/min, C-G) or end-stage renal disease (ESRD, ClCr < 15 mL/min, C-G) is 25 mg every day or 50 mg every other day. Supplemental doses should not be given to patients after dialysis [see Use in Specific Populations (8.6) and Clinical Pharmacology (12.3)].
2.3 Dosage Recommendations for Patients with Hepatic Impairment
The recommended dose in patients with moderate to severe hepatic impairment (Child-Pugh score 7 to 15) is 50 mg per day. Dose escalation above 100 mg per day is not recommended [see Use in Specific Populations (8.7) and Clinical Pharmacology (12.3)].
2.4 Maintenance/Continuation/Extended Treatment
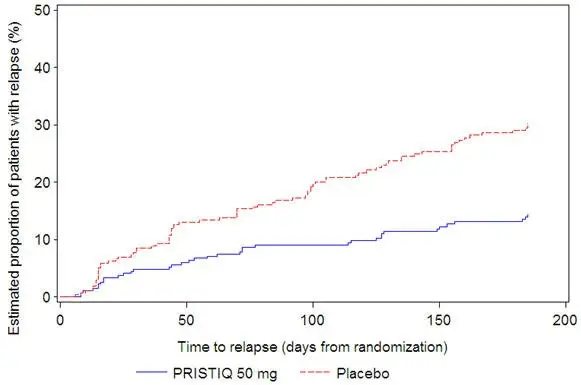
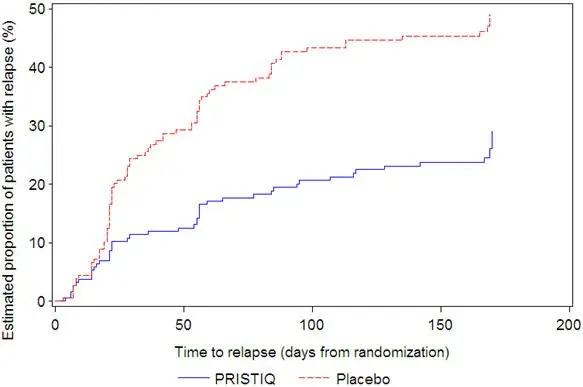
It is generally agreed that acute episodes of major depressive disorder require several months or longer of sustained pharmacologic therapy. Longer-term efficacy of PRISTIQ (50–400 mg) was established in two maintenance trials [see Clinical Studies (14)]. Patients should be periodically reassessed to determine the need for continued treatment.
2.5 Discontinuing PRISTIQ
Adverse reactions may occur upon discontinuation of PRISTIQ [see Warnings and Precautions (5.7)]. Gradually reduce the dosage rather than stopping PRISTIQ abruptly when discontinuing therapy with PRISTIQ. In some patients, discontinuation may need to occur over a period of several months.
2.6 Switching Patients From Other Antidepressants to PRISTIQ
Discontinuation symptoms have been reported when switching patients from other antidepressants, including venlafaxine, to PRISTIQ. Tapering of the initial antidepressant may be necessary to minimize discontinuation symptoms.
2.7 Switching Patients to or from a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with PRISTIQ. Conversely, at least 7 days should be allowed after stopping PRISTIQ before starting an MAOI intended to treat psychiatric disorders [see Contraindications (4)].
2.8 Use of PRISTIQ with other MAOIs such as Linezolid or Methylene Blue
Do not start PRISTIQ in a patient who is being treated with linezolid or intravenous methylene blue because there is increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered [see Contraindications (4)].
In some cases, a patient already receiving PRISTIQ therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, PRISTIQ should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 7 days or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with PRISTIQ may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue [see Warnings and Precautions (5.2)].
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with PRISTIQ is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use [see Warnings and Precautions (5.2)].
3. Dosage Forms and Strengths
- 25 mg Tablet: tan, square pyramid tablet debossed with "W" over "25" on the flat side
- 50 mg Tablet: light pink, square pyramid tablet debossed with "W" over "50" on the flat side
- 100 mg Tablet: reddish-orange, square pyramid tablet debossed with "W" over "100" on the flat side
4. Contraindications
- Hypersensitivity to desvenlafaxine succinate, venlafaxine hydrochloride or to any excipients in the PRISTIQ formulation. Angioedema has been reported in patients treated with PRISTIQ [see Adverse Reactions (6.1)].
- The use of MAOIs intended to treat psychiatric disorders with PRISTIQ or within 7 days of stopping treatment with PRISTIQ is contraindicated because of an increased risk of serotonin syndrome. The use of PRISTIQ within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated [see Dosage and Administration (2.7) and Warnings and Precautions (5.2)].
- Starting PRISTIQ in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is also contraindicated because of an increased risk of serotonin syndrome [see Dosage and Administration (2.8) and Warnings and Precautions (5.2)].
5. Warnings and Precautions
5.1 Suicidal Thoughts and Behaviors in Pediatric and Young Adult Patients
Patients with MDD, both adult and pediatric, may experience worsening of their depression and/or the emergence of suicidal ideation and behavior (suicidality) or unusual changes in behavior, whether or not they are taking antidepressant medications, and this risk may persist until significant remission occurs. Suicide is a known risk of depression and certain other psychiatric disorders, and these disorders themselves are the strongest predictors of suicide. There has been a long-standing concern, however, that antidepressants may have a role in inducing worsening of depression and the emergence of suicidality in certain patients during the early phases of treatment. Pooled analyses of short-term placebo-controlled studies of antidepressant drugs (SSRIs and others) showed that these drugs increase the risk of suicidal thinking and behavior (suicidality) in children, adolescents, and young adults (ages 18 to 24) with major depressive disorder (MDD) and other psychiatric disorders. Short-term studies did not show an increase in the risk of suicidality with antidepressants compared to placebo in adults beyond age 24; there was a reduction with antidepressants compared to placebo in adults aged 65 and older.
The pooled analyses of placebo-controlled studies in children and adolescents with MDD, obsessive compulsive disorder (OCD), or other psychiatric disorders included a total of 24 short-term studies of 9 antidepressant drugs in over 4,400 patients. The pooled analyses of placebo-controlled studies in adults with MDD or other psychiatric disorders included a total of 295 short-term studies (median duration of 2 months) of 11 antidepressant drugs in over 77,000 patients. There was considerable variation in risk of suicidality among drugs, but a tendency toward an increase in the younger patients for almost all drugs studied. There were differences in absolute risk of suicidality across the different indications, with the highest incidence in MDD. The risk differences (drug vs. placebo), however, were relatively stable within age strata and across indications. These risk differences (drug-placebo difference in the number of cases of suicidality per 1,000 patients treated) are provided in Table 1.
| Age Range | Drug-Placebo Difference in Number of Cases of Suicidality per 1,000 Patients Treated |
|---|---|
| Increases Compared to Placebo | |
| <18 | 14 additional cases |
| 18 to 24 | 5 additional cases |
| Decreases Compared to Placebo | |
| 25 to 64 | 1 fewer case |
| ≥65 | 6 fewer cases |
No suicides occurred in any of the pediatric studies. There were suicides in the adult studies, but the number was not sufficient to reach any conclusion about drug effect on suicide.
It is unknown whether the suicidality risk extends to longer-term use, i.e., beyond several months. However, there is substantial evidence from placebo-controlled maintenance studies in adults with depression that the use of antidepressants can delay the recurrence of depression.
All patients being treated with antidepressants for any indication should be monitored appropriately and observed closely for clinical worsening, suicidality, and unusual changes in behavior, especially during the initial few months of a course of drug therapy, or at times of dose changes, either increases or decreases.
The following symptoms, anxiety, agitation, panic attacks, insomnia, irritability, hostility, aggressiveness, impulsivity, akathisia (psychomotor restlessness), hypomania, and mania, have been reported in adult and pediatric patients being treated with antidepressants for major depressive disorder as well as for other indications, both psychiatric and nonpsychiatric. Although a causal link between the emergence of such symptoms and either the worsening of depression and/or the emergence of suicidal impulses has not been established, there is concern that such symptoms may represent precursors to emerging suicidality.
Consideration should be given to changing the therapeutic regimen, including possibly discontinuing the medication, in patients whose depression is persistently worse, or who are experiencing emergent suicidality or symptoms that might be precursors to worsening depression or suicidality, especially if these symptoms are severe, abrupt in onset, or were not part of the patient's presenting symptoms.
If the decision has been made to discontinue treatment, medication should be tapered, as rapidly as is feasible, but with recognition that abrupt discontinuation can be associated with certain symptoms [see Dosage and Administration (2.4), Warnings and Precautions (5.7)].
Families and caregivers of patients being treated with antidepressants for major depressive disorder or other indications, both psychiatric and nonpsychiatric, should be alerted about the need to monitor patients for the emergence of agitation, irritability, unusual changes in behavior, and the other symptoms described above, as well as the emergence of suicidality, and to report such symptoms immediately to healthcare providers. Such monitoring should include daily observation by families and caregivers.
Prescriptions for PRISTIQ should be written for the smallest quantity of tablets consistent with good patient management, in order to reduce the risk of overdose.
5.2 Serotonin Syndrome
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and selective-serotonin reuptake inhibitors (SSRIs), including PRISTIQ, can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John's Wort) and with drugs that impair metabolism of serotonin, i.e., MAOIs [see Contraindications (4), Drug Interactions (7.1)]. Serotonin syndrome can also occur when these drugs are used alone.
Serotonin syndrome signs and symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of PRISTIQ with MAOIs is contraindicated. In addition, do not initiate PRISTIQ in a patient being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking PRISTIQ, discontinue PRISTIQ before initiating treatment with the MAOI [see Contraindications (4), Drug Interactions (7.1)].
Monitor all patients taking PRISTIQ for the emergence of serotonin syndrome. Discontinue treatment with PRISTIQ and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of PRISTIQ with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms.
5.3 Elevated Blood Pressure
Patients receiving PRISTIQ should have regular monitoring of blood pressure since increases in blood pressure were observed in clinical studies [see Adverse Reactions (6.1)]. Pre-existing hypertension should be controlled before initiating treatment with PRISTIQ. Caution should be exercised in treating patients with pre-existing hypertension, cardiovascular, or cerebrovascular conditions that might be compromised by increases in blood pressure. Cases of elevated blood pressure requiring immediate treatment have been reported with PRISTIQ.
Sustained blood pressure increases could have adverse consequences. For patients who experience a sustained increase in blood pressure while receiving PRISTIQ, either dose reduction or discontinuation should be considered [see Adverse Reactions (6.1)].
5.4 Increased Risk of Bleeding
Drugs that interfere with serotonin reuptake inhibition, including PRISTIQ, may increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs, warfarin, and other anticoagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Bleeding events related to SSRIs and SNRIs have ranged from ecchymosis, hematoma, epistaxis, and petechiae to life-threatening hemorrhages. Inform patients about the risk of bleeding associated with the concomitant use of PRISTIQ and antiplatelet agents or anticoagulants. For patients taking warfarin, carefully monitor coagulation indices when initiating, titrating, or discontinuing PRISTIQ.
5.5 Angle Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including PRISTIQ may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Avoid use of antidepressants, including PRISTIQ, in patients with untreated anatomically narrow angles.
5.6 Activation of Mania/Hypomania
During all MDD phase 2 and phase 3 studies, mania was reported for approximately 0.02% of patients treated with PRISTIQ. Activation of mania/hypomania has also been reported in a small proportion of patients with major affective disorder who were treated with other marketed antidepressants. As with all antidepressants, PRISTIQ should be used cautiously in patients with a history or family history of mania or hypomania.
5.7 Discontinuation Syndrome
Adverse reactions after discontinuation of serotonergic antidepressants, particularly after abrupt discontinuation, include: nausea, sweating, dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesia, such as electric shock sensations), tremor, anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures [see Adverse Reactions (6.1)].
There have been postmarketing reports of serious discontinuation symptoms with PRISTIQ, which can be protracted and severe. Completed suicide, suicidal thoughts, and severe aggression (including hostility, rage, and homicidal ideation) have been observed in patients during reduction in PRISTIQ dosage, including during discontinuation. Other postmarketing reports describe visual changes (such as blurred vision or trouble focusing) and increased blood pressure after stopping or reducing the dose of PRISTIQ.
Patients should be monitored when discontinuing treatment with PRISTIQ. A gradual reduction in the dose, rather than abrupt cessation, is recommended. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the healthcare provider may continue decreasing the dose, but at a more gradual rate. In some patients, discontinuation may need to occur over a period of several months [see Dosage and Administration (2.5)].
5.8 Seizure
Cases of seizure have been reported in pre-marketing clinical studies with PRISTIQ. PRISTIQ has not been systematically evaluated in patients with a seizure disorder. Patients with a history of seizures were excluded from pre-marketing clinical studies. PRISTIQ should be prescribed with caution in patients with a seizure disorder.
5.9 Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs and SNRIs, including PRISTIQ. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases with serum sodium lower than 110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SSRIs and SNRIs. Also, patients taking diuretics or who are otherwise volume depleted can be at greater risk [see Use in Specific Populations (8.5) and Clinical Pharmacology (12.3)]. Discontinuation of PRISTIQ should be considered in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which can lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
5.10 Interstitial Lung Disease and Eosinophilic Pneumonia
Interstitial lung disease and eosinophilic pneumonia associated with venlafaxine (the parent drug of PRISTIQ) therapy have been rarely reported. The possibility of these adverse events should be considered in patients treated with PRISTIQ who present with progressive dyspnea, cough, or chest discomfort. Such patients should undergo a prompt medical evaluation, and discontinuation of PRISTIQ should be considered.
5.11 Sexual Dysfunction
Use of SNRIs, including PRISTIQ, may cause symptoms of sexual dysfunction [see Adverse Reactions (6.1)]. In male patients, SNRI use may result in ejaculatory delay or failure, decreased libido, and erectile dysfunction. In female patients, SNRI use may result in decreased libido and delayed or absent orgasm.
It is important for prescribers to inquire about sexual function prior to initiation of PRISTIQ and to inquire specifically about changes in sexual function during treatment, because sexual function may not be spontaneously reported. When evaluating changes in sexual function, obtaining a detailed history (including timing of symptom onset) is important because sexual symptoms may have other causes, including the underlying psychiatric disorder. Discuss potential management strategies to support patients in making informed decisions about treatment.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in greater detail in other sections of the label.
- Hypersensitivity [see Contraindications (4)]
- Suicidal Thoughts and Behaviors in Pediatric and Young Adult Patients [see Warnings and Precautions (5.1)]
- Serotonin Syndrome [see Warnings and Precautions (5.2)]
- Elevated Blood Pressure [see Warnings and Precautions (5.3)]
- Increased Risk of Bleeding [see Warnings and Precautions (5.4)]
- Angle Closure Glaucoma [see Warnings and Precautions (5.5)]
- Activation of Mania/Hypomania [see Warnings and Precautions (5.6)]
- Discontinuation Syndrome [see Warnings and Precautions (5.7)]
- Seizure [see Warnings and Precautions (5.8)]
- Hyponatremia [see Warnings and Precautions (5.9)]
- Interstitial Lung Disease and Eosinophilic Pneumonia [see Warnings and Precautions (5.10)]
- Sexual Dysfunction [see Warnings and Precautions (5.11)]
6.1 Clinical Studies Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in clinical practice.
Laboratory, ECG and Vital Sign Changes Observed in MDD Clinical Studies
The following changes were observed in pre-marketing placebo-controlled, short-term MDD studies with PRISTIQ.
Proteinuria
Proteinuria, greater than or equal to trace, was observed in the pre-marketing fixed-dose controlled studies (see Table 5). This proteinuria was not associated with increases in BUN or creatinine and was generally transient.
| PRISTIQ | |||||
|---|---|---|---|---|---|
| Placebo | 50 mg | 100 mg | 200 mg | 400 mg | |
| Proteinuria | 4 | 6 | 8 | 5 | 7 |
Vital Sign Changes
Table 6 summarizes the changes that were observed in placebo-controlled, short-term, pre-marketing studies with PRISTIQ in patients with MDD (doses 50 to 400 mg).
| PRISTIQ | |||||
|---|---|---|---|---|---|
| Placebo | 50 mg | 100 mg | 200 mg | 400 mg | |
| Blood pressure | |||||
| Supine systolic bp (mm Hg) | -1.4 | 1.2 | 2.0 | 2.5 | 2.1 |
| Supine diastolic bp (mm Hg) | -0.6 | 0.7 | 0.8 | 1.8 | 2.3 |
| Pulse rate | |||||
| Supine pulse (bpm) | -0.3 | 1.3 | 1.3 | 0.9 | 4.1 |
| Weight (kg) | 0.0 | -0.4 | -0.6 | -0.9 | -1.1 |
Treatment with PRISTIQ at all doses from 50 mg per day to 400 mg per day in controlled studies was associated with sustained hypertension, defined as treatment-emergent supine diastolic blood pressure (SDBP) ≥90 mm Hg and ≥10 mm Hg above baseline for 3 consecutive on-therapy visits (see Table 7). Analyses of patients in PRISTIQ pre-marketing short-term controlled studies who met criteria for sustained hypertension revealed a consistent increase in the proportion of patients who developed sustained hypertension. This was seen at all doses with a suggestion of a higher rate at 400 mg per day.
| Treatment Group | Proportion of Patients with Sustained Hypertension |
|---|---|
| Placebo | 0.5% |
| PRISTIQ 50 mg per day | 1.3% |
| PRISTIQ 100 mg per day | 0.7% |
| PRISTIQ 200 mg per day | 1.1% |
| PRISTIQ 400 mg per day | 2.3% |
6.2 Postmarketing Experience
The following adverse reaction has been identified during post-approval use of PRISTIQ. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
Skin and subcutaneous tissue disorders – Stevens-Johnson syndrome
Gastrointestinal disorders – Pancreatitis acute
Cardiovascular system – Takotsubo cardiomyopathy
7. Drug Interactions
7.1 Drugs Having Clinically Important Interactions with PRISTIQ
| Monoamine Oxidase Inhibitors (MAOI) | |
| Clinical Impact | The concomitant use of SSRIs and SNRIs including PRISTIQ with MAOIs increases the risk of serotonin syndrome. |
| Intervention | Concomitant use of PRISTIQ is contraindicated:
|
| Examples | selegiline, tranylcypromine, isocarboxazid, phenelzine, linezolid, methylene blue |
| Other Serotonergic Drugs | |
| Clinical Impact | Concomitant use of PRISTIQ with other serotonergic drugs increases the risk of serotonin syndrome. |
| Intervention | Monitor for symptoms of serotonin syndrome when PRISTIQ is used concomitantly with other drugs that may affect the serotonergic neurotransmitter systems. If serotonin syndrome occurs, consider discontinuation of PRISTIQ and/or concomitant serotonergic drugs [see Warnings and Precautions (5.2)]. |
| Examples | other SNRIs, SSRIs, triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, buspirone, amphetamines, tryptophan, and St. John's Wort |
| Drugs that Interfere with Hemostasis | |
| Clinical Impact | Concomitant use of PRISTIQ with an antiplatelet or anticoagulant drug may potentiate the risk of bleeding. This may be due to the effect of PRISTIQ on the release of serotonin by platelets. |
| Intervention | Closely monitor for bleeding for patients receiving an antiplatelet or anticoagulant drug when PRISTIQ is initiated or discontinued [see Warnings and Precautions (5.4)]. |
| Examples | NSAIDs, aspirin, and warfarin |
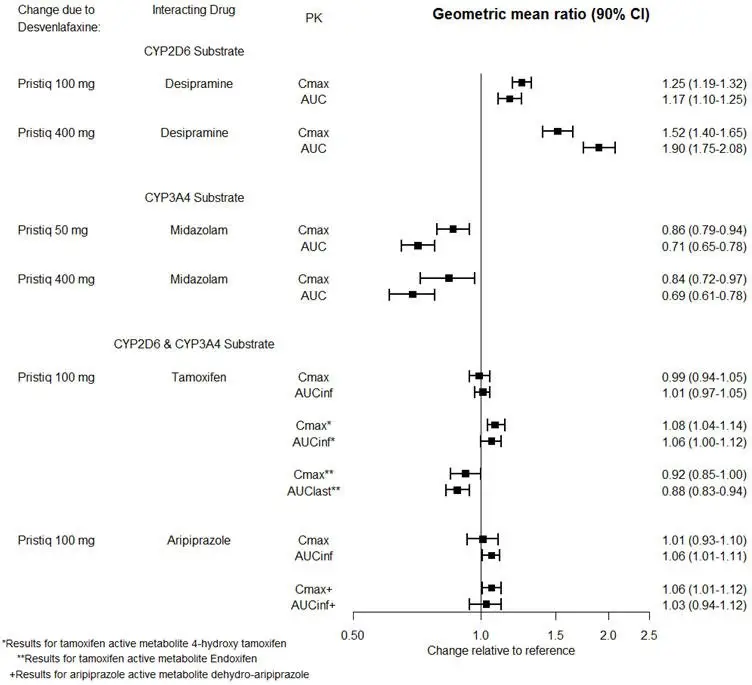
| Drugs that are Primarily Metabolized by CYP2D6 | |
| Clinical Impact | Concomitant use of PRISTIQ increases Cmax and AUC of a drug primarily metabolized by CYP2D6 which may increase the risk of toxicity of the CYP2D6 substrate drug [see Clinical Pharmacology (12.3)]. |
| Intervention | Original dose should be taken when co-administered with PRISTIQ 100 mg or lower. Reduce the dose of these drugs by up to one-half if co-administered with 400 mg of PRISTIQ. |
| Examples | desipramine, atomoxetine, dextromethorphan, metoprolol, nebivolol, perphenazine, tolterodine |
7.2 Drugs Having No Clinically Important Interactions with PRISTIQ
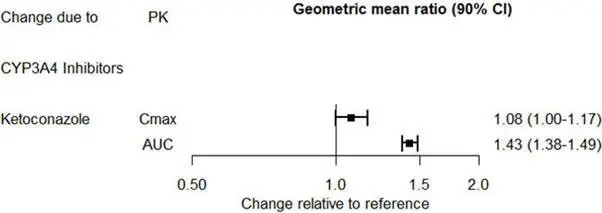
Based on pharmacokinetic studies, no dosage adjustment is required for drugs that are mainly metabolized by CYP3A4 (e.g., midazolam), or for drugs that are metabolized by both CYP2D6 and CYP3A4 (e.g., tamoxifen, aripiprazole), when administered concomitantly with PRISTIQ [see Clinical Pharmacology (12.3)].
7.3 Alcohol
A clinical study has shown that PRISTIQ does not increase the impairment of mental and motor skills caused by ethanol. However, as with all CNS-active drugs, patients should be advised to avoid alcohol consumption while taking PRISTIQ.
7.4 Drug-Laboratory Test Interactions
False-positive urine immunoassay screening tests for phencyclidine (PCP) and amphetamine have been reported in patients taking desvenlafaxine. This is due to lack of specificity of the screening tests. False positive test results may be expected for several days following discontinuation of desvenlafaxine therapy. Confirmatory tests, such as gas chromatography/mass spectrometry, will distinguish desvenlafaxine from PCP and amphetamine.
8. Use In Specific Populations
8.2 Lactation
Data
A lactation study was conducted in 10 breastfeeding women (at a mean of 4.3 months post-partum) who were being treated with a 50–150 mg daily dose of desvenlafaxine for postpartum depression. Sampling was performed at steady state (up to 8 samples) over a 24 hour dosing period, and included foremilk and hindmilk. The mean relative infant dose was calculated to be 6.8% (range of 5.5–8.1%). No adverse reactions were seen in the infants.
8.4 Pediatric Use
The safety and effectiveness of PRISTIQ have not been established in pediatric patients for the treatment of MDD.
Efficacy was not demonstrated in two adequate and well controlled, 8-week, randomized, double-blind, placebo-controlled, parallel group studies conducted in 587 patients (7 to 17 years of age) for the treatment of MDD.
Antidepressants, such as PRISTIQ, increase the risk of suicidal thoughts and behaviors in pediatric patients [see the Boxed Warning and Warnings and Precautions (5.1)].
PRISTIQ was associated with a decrease in body weight in placebo-controlled trials in pediatric patients with MDD. The incidence of weight loss (≥3.5% of baseline weight) was 22%, 14%, and 7% for patients treated with low dose PRISTIQ, high dose PRISTIQ, and placebo, respectively.
The risks associated with longer term PRISTIQ use were assessed in 6-month, open-label extension studies in pediatric patients (7 to 17 years of age) with MDD. Pediatric patients (7 to 17 years of age) had mean changes in weight that approximated expected changes, based on data from age- and sex-matched peers.
In clinical trials, when compared to adult patients receiving the same dose of PRISTIQ, exposure to desvenlafaxine was similar in adolescent patients 12 to 17 years of age, and was about 30% higher in pediatric patients 7 to 11 years of age.
8.5 Geriatric Use
Of the 4,158 patients in pre-marketing clinical studies with PRISTIQ, 6% were 65 years of age or older. No overall differences in safety or efficacy were observed between these patients and younger patients; however, in the short-term placebo-controlled studies, there was a higher incidence of systolic orthostatic hypotension in patients ≥65 years of age compared to patients <65 years of age treated with PRISTIQ [see Adverse Reactions (6.1)]. For elderly patients, possible reduced renal clearance of PRISTIQ should be considered when determining dose [see Dosage and Administration (2.2) and Clinical Pharmacology (12.3)].
SSRIs and SNRIs, including PRISTIQ, have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse event [see Warnings and Precautions (5.9)].
10. Overdosage
10.1 Human Experience with Overdosage
There is limited clinical trial experience with desvenlafaxine succinate overdosage in humans. However, desvenlafaxine (PRISTIQ) is the major active metabolite of venlafaxine. Overdose experience reported with venlafaxine (the parent drug of PRISTIQ) is presented below; the identical information can be found in the Overdosage section of the venlafaxine package insert.
In postmarketing experience, overdose with venlafaxine (the parent drug of PRISTIQ) has occurred predominantly in combination with alcohol and/or other drugs. The most commonly reported events in overdosage include tachycardia, changes in level of consciousness (ranging from somnolence to coma), mydriasis, seizures, and vomiting. Electrocardiogram changes (e.g., prolongation of QT interval, bundle branch block, QRS prolongation), sinus and ventricular tachycardia, bradycardia, hypotension, rhabdomyolysis, vertigo, liver necrosis, serotonin syndrome, and death have been reported.
Published retrospective studies report that venlafaxine overdosage may be associated with an increased risk of fatal outcomes compared to that observed with SSRI antidepressant products, but lower than that for tricyclic antidepressants. Epidemiological studies have shown that venlafaxine-treated patients have a higher pre-existing burden of suicide risk factors than SSRI-treated patients. The extent to which the finding of an increased risk of fatal outcomes can be attributed to the toxicity of venlafaxine in overdosage, as opposed to some characteristic(s) of venlafaxine-treated patients, is not clear.
11. Pristiq Description
PRISTIQ is an extended-release tablet for oral administration that contains desvenlafaxine succinate, a structurally novel SNRI for the treatment of MDD. Desvenlafaxine (O-desmethylvenlafaxine) is the major active metabolite of the antidepressant venlafaxine, a medication used to treat major depressive disorder.
Desvenlafaxine is designated RS-4-[2-dimethylamino-1-(1-hydroxycyclohexyl)ethyl]phenol and has the empirical formula of C16H25NO2 (free base) and C16H25NO2∙C4H6O4∙H2O (succinate monohydrate). Desvenlafaxine succinate monohydrate has a molecular weight of 399.48. The structural formula is shown below.
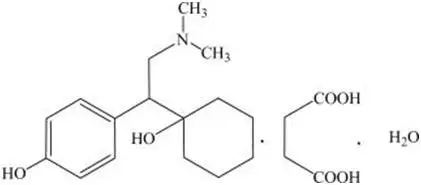
Desvenlafaxine succinate is a white to off-white powder that is soluble in water. The solubility of desvenlafaxine succinate is pH dependent. Its octanol:aqueous system (at pH 7.0) partition coefficient is 0.21.
PRISTIQ is formulated as an extended-release tablet for once-a-day oral administration.
Each tablet contains 38 mg, 76 mg or 152 mg of desvenlafaxine succinate equivalent to 25 mg, 50 mg or 100 mg of desvenlafaxine, respectively.
Inactive ingredients for the 25 mg tablet consist of hypromellose, microcrystalline cellulose, talc, magnesium stearate, a film coating which consists of polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, and iron oxides.
Inactive ingredients for the 50 mg tablet consist of hypromellose, microcrystalline cellulose, talc, magnesium stearate and film coating, which consists of polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, and iron oxides.
Inactive ingredients for the 100 mg tablet consist of hypromellose, microcrystalline cellulose, talc, magnesium stearate and film coating, which consists of polyvinyl alcohol, polyethylene glycol, talc, titanium dioxide, iron oxide and FD&C yellow #6.
12. Pristiq - Clinical Pharmacology
12.1 Mechanism of Action
The exact mechanism of the antidepressant action of desvenlafaxine is unknown, but is thought to be related to the potentiation of serotonin and norepinephrine in the central nervous system, through inhibition of their reuptake. Non-clinical studies have shown that desvenlafaxine is a potent and selective SNRI.
12.2 Pharmacodynamics
Desvenlafaxine lacked significant affinity for numerous receptors, including muscarinic-cholinergic, H1-histaminergic, or α1-adrenergic receptors in vitro. Desvenlafaxine also lacked monoamine oxidase (MAO) inhibitory activity.
12.3 Pharmacokinetics
The single-dose pharmacokinetics of desvenlafaxine are linear and dose-proportional in a dose range of 50 to 600 mg (1 to 12 times the recommended approved dosage) per day. With once-daily dosing, steady-state plasma concentrations are achieved within approximately 4 to 5 days. At steady-state, multiple-dose accumulation of desvenlafaxine is linear and predictable from the single-dose pharmacokinetic profile.
16. How is Pristiq supplied
PRISTIQ® (desvenlafaxine) extended-release tablets are available as follows:
25 mg, tan, square pyramid tablet debossed with "W" (over) "25" on the flat side
- NDC 0008-1210-30, bottle of 30 tablets in unit-of-use package
50 mg, light pink, square pyramid tablet debossed with "W" (over) "50" on the flat side
- NDC 0008-1211-14, bottle of 14 tablets in unit-of-use package
- NDC 0008-1211-30, bottle of 30 tablets in unit-of-use package
- NDC 0008-1211-01, bottle of 90 tablets in unit-of-use package
- NDC 0008-1211-50, 10 blisters of 10 (HUD)
100 mg, reddish-orange, square pyramid tablet debossed with "W" (over) "100" on the flat side
- NDC 0008-1222-14, bottle of 14 tablets in unit-of-use package
- NDC 0008-1222-30, bottle of 30 tablets in unit-of-use package
- NDC 0008-1222-01, bottle of 90 tablets in unit-of-use package
- NDC 0008-1222-50, 10 blisters of 10 (HUD)
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 11/2021 | |||
| MEDICATION GUIDE PRISTIQ® (pris-TEEK) (desvenlafaxine) extended-release tablets |
||||
| What is the most important information I should know about PRISTIQ? PRISTIQ can cause serious side effects, including:
|
||||
|
|
|||
What is PRISTIQ?
|
||||
Do not take PRISTIQ if you:
|
||||
Before taking PRISTIQ tell your healthcare provider about all your medical conditions, including if you:
|
||||
|
||||
PRISTIQ and other medicines may affect each other causing possible serious side effects. PRISTIQ may affect the way other medicines work and other medicines may affect the way PRISTIQ works. Especially tell your healthcare provider if you take:
Do not start or stop any other medicines during treatment with PRISTIQ without talking to your healthcare provider first. Stopping PRISTIQ suddenly may cause you to have serious side effects. See, "What are the possible side effects of PRISTIQ?" Know the medicines you take. Keep a list of them to show to your healthcare providers when you get a new medicine. |
||||
How should I take PRISTIQ?
|
||||
What should I avoid while taking PRISTIQ?
|
||||
| What are the possible side effects of PRISTIQ?
PRISTIQ can cause serious side effects, including:
|
||||
|
|
|||
|
||||
|
|
|
||
|
||||
|
|
|||
| These are not all the possible side effects of PRISTIQ. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||||
How should I store PRISTIQ?
|
||||
| General Information about the safe and effective use of PRISTIQ
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not take PRISTIQ for a condition for which it was not prescribed. Do not give PRISTIQ to other people, even if they have the same symptoms that you have. It may harm them. You can ask your pharmacist or healthcare provider for information about PRISTIQ that is written for healthcare professionals. |
||||
| What are the ingredients in PRISTIQ? Active ingredient: desvenlafaxine Inactive ingredients:
This product's label may have been updated. For current full prescribing information, please visit www.pfizer.com.
LAB-0539-13.0 For more information, go to www.pristiq.com or call 1-888-PRISTIQ (774-7847). |
||||
| PRISTIQ
EXTENDED-RELEASE
desvenlafaxine succinate tablet, extended release |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PRISTIQ
EXTENDED-RELEASE
desvenlafaxine succinate tablet, extended release |
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||
| PRISTIQ
EXTENDED-RELEASE
desvenlafaxine succinate tablet, extended release |
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
|
||||||||||||||||||||||
| Labeler - Wyeth Pharmaceuticals LLC, a subsidiary of Pfizer Inc. (113008515) |
| Registrant - Pfizer Inc (113480771) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Pharmaceuticals LLC | 829084552 | PACK(0008-1210, 0008-1211, 0008-1222) , LABEL(0008-1210, 0008-1211, 0008-1222) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Asia Manufacturing Pte Ltd | 936889401 | ANALYSIS(0008-1210, 0008-1211, 0008-1222) , API MANUFACTURE(0008-1210, 0008-1211, 0008-1222) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 986019327 | ANALYSIS(0008-1210, 0008-1211, 0008-1222) , MANUFACTURE(0008-1210, 0008-1211, 0008-1222) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pfizer Ireland Pharmaceuticals | 985052076 | ANALYSIS(0008-1210, 0008-1211, 0008-1222) , API MANUFACTURE(0008-1210, 0008-1211, 0008-1222) | |