Drug Detail:Procrit (Epoetin alfa [ e-poe-e-tin-al-fa ])
Drug Class: Recombinant human erythropoietins
Highlights of Prescribing Information
PROCRIT® (epoetin alfa) injection, for intravenous or subcutaneous use
Initial U.S. Approval: 1989
WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE
See full prescribing information for complete boxed warning.
Chronic Kidney Disease:
- In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL (5.1).
- No trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks (2.2).
- Use the lowest PROCRIT dose sufficient to reduce the need for red blood cell (RBC) transfusions (5.1).
Cancer:
- ESAs shortened overall survival and/or increased the risk of tumor progression or recurrence in clinical studies of patients with breast, non-small cell lung, head and neck, lymphoid, and cervical cancers (5.2).
- Use the lowest dose to avoid RBC transfusions (2.4).
- Use ESAs only for anemia from myelosuppressive chemotherapy (1.3).
- ESAs are not indicated for patients receiving myelosuppressive chemotherapy when the anticipated outcome is cure (1.5).
- Discontinue following the completion of a chemotherapy course (2.4).
Perisurgery:
- Due to increased risk of deep venous thrombosis (DVT), DVT prophylaxis is recommended (5.1).
Recent Major Changes
| Dosage and Administration, Patients with Chronic Kidney Disease (2.2) | 9/2017 |
| Warnings and Precautions, Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients With Cancer (5.2) | 7/2018 |
| Warnings and Precautions, Severe Cutaneous Reactions (5.8) | 9/2017 |
| Warnings and Precautions, Risk of Serious Adverse Reactions Due to Benzyl Alcohol Preservative (5.9) | 9/2017 |
Indications and Usage for Procrit
PROCRIT is an erythropoiesis-stimulating agent (ESA) indicated for:
- Treatment of anemia due to
- -
- Chronic Kidney Disease (CKD) in patients on dialysis and not on dialysis (1.1).
- -
- Zidovudine in patients with HIV-infection (1.2).
- -
- The effects of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy (1.3).
- Reduction of allogeneic RBC transfusions in patients undergoing elective, noncardiac, nonvascular surgery (1.4).
Limitations of Use
PROCRIT has not been shown to improve quality of life, fatigue, or patient well-being (1.5).
PROCRIT is not indicated for use:
- In patients with cancer receiving hormonal agents, biologic products, or radiotherapy, unless also receiving concomitant myelosuppressive chemotherapy (1.5).
- In patients with cancer receiving myelosuppressive chemotherapy when the anticipated outcome is cure (1.5).
- In patients with cancer receiving myelosuppressive chemotherapy in whom the anemia can be managed by transfusion (1.5).
- In patients scheduled for surgery who are willing to donate autologous blood (1.5).
- In patients undergoing cardiac or vascular surgery (1.5).
- As a substitute for RBC transfusions in patients who require immediate correction of anemia (1.5).
Procrit Dosage and Administration
- Evaluate iron status before and during treatment and maintain iron repletion. Correct or exclude other causes of anemia before initiating treatment (2.1).
- In pregnant women, lactating women, neonates, infants: Use only single-dose vials (2.1).
- Patients with CKD: Initial dose: 50 to 100 Units/kg 3 times weekly (adults) and 50 Units/kg 3 times weekly (pediatric patients). Individualize maintenance dose. Intravenous route recommended for patients on hemodialysis (2.2).
- Patients on Zidovudine due to HIV-infection: 100 Units/kg 3 times weekly (2.3).
- Patients with Cancer on Chemotherapy: 40,000 Units weekly or 150 Units/kg 3 times weekly (adults); 600 Units/kg intravenously weekly (pediatric patients ≥ 5 years) (2.4).
- Surgery Patients: 300 Units/kg per day daily for 15 days or 600 Units/kg weekly (2.5).
Dosage Forms and Strengths
- Injection
- 2,000 Units/mL, 3,000 Units/mL, 4,000 Units/mL, 10,000 Units/mL, and 40,000 Units/mL in single-dose vials (3)
- 20,000 Units/2 mL (10,000 Units/mL) and 20,000 Units/mL in multiple-dose vials containing benzyl alcohol (3)
Contraindications
- Uncontrolled hypertension (4)
- Pure red cell aplasia (PRCA) that begins after treatment with PROCRIT or other erythropoietin protein drugs (4)
- Serious allergic reactions to PROCRIT (4)
- Use of the multiple-dose vials containing benzyl alcohol in neonates, infants, pregnant women, and lactating women (4)
Warnings and Precautions
- Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism: Using ESAs to target a hemoglobin level of greater than 11 g/dL increases the risk of serious adverse cardiovascular reactions and has not been shown to provide additional benefit (5.1 and 14.1). Use caution in patients with coexistent cardiovascular disease and stroke (5.1).
- Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients With Cancer (5.2).
- Hypertension: Control hypertension prior to initiating and during treatment with PROCRIT (5.3).
- Seizures: PROCRIT increases the risk for seizures in patients with CKD (5.4). Increase monitoring of these patients for changes in seizure frequency or premonitory symptoms (5.4).
- PRCA: If severe anemia and low reticulocyte count develop during PROCRIT treatment, withhold PROCRIT and evaluate for PRCA (5.6).
- Serious Allergic Reactions: Discontinue PROCRIT and manage reactions (5.7).
- Severe Cutaneous Reactions: Discontinue PROCRIT (5.8).
Adverse Reactions/Side Effects
- Patients with CKD: Adverse reactions in ≥ 5% of PROCRIT-treated patients in clinical studies were hypertension, arthralgia, muscle spasm, pyrexia, dizziness, medical device malfunction, vascular occlusion, and upper respiratory tract infection (6.1).
- Patients on Zidovudine due to HIV-infection: Adverse reactions in ≥ 5% of PROCRIT-treated patients in clinical studies were pyrexia, cough, rash, and injection site irritation (6.1).
- Patients with Cancer on Chemotherapy: Adverse reactions in ≥ 5% of PROCRIT-treated patients in clinical studies were nausea, vomiting, myalgia, arthralgia, stomatitis, cough, weight decrease, leukopenia, bone pain, rash, hyperglycemia, insomnia, headache, depression, dysphagia, hypokalemia, and thrombosis (6.1).
- Surgery Patients: Adverse reactions in ≥ 5% of PROCRIT-treated patients in clinical studies were nausea, vomiting, pruritus, headache, injection site pain, chills, deep vein thrombosis, cough, and hypertension (6.1).
To report SUSPECTED ADVERSE REACTIONS, contact Janssen Products, LP at 1-800-JANSSEN (1-800-526-7736) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 7/2018
Related/similar drugs
ferrous sulfate, pyridoxine, Revlimid, FeroSul, lenalidomide, Aranesp, RetacritFull Prescribing Information
WARNING: ESAs INCREASE THE RISK OF DEATH, MYOCARDIAL INFARCTION, STROKE, VENOUS THROMBOEMBOLISM, THROMBOSIS OF VASCULAR ACCESS AND TUMOR PROGRESSION OR RECURRENCE
1. Indications and Usage for Procrit
1.1 Anemia Due to Chronic Kidney Disease
PROCRIT is indicated for the treatment of anemia due to chronic kidney disease (CKD), including patients on dialysis and not on dialysis to decrease the need for red blood cell (RBC) transfusion.
1.2 Anemia Due to Zidovudine in Patients with HIV-infection
PROCRIT is indicated for the treatment of anemia due to zidovudine administered at ≤ 4200 mg/week in patients with HIV-infection with endogenous serum erythropoietin levels of ≤ 500 mUnits/mL.
1.3 Anemia Due to Chemotherapy in Patients With Cancer
PROCRIT is indicated for the treatment of anemia in patients with non-myeloid malignancies where anemia is due to the effect of concomitant myelosuppressive chemotherapy, and upon initiation, there is a minimum of two additional months of planned chemotherapy.
1.4 Reduction of Allogeneic Red Blood Cell Transfusions in Patients Undergoing Elective, Noncardiac, Nonvascular Surgery
PROCRIT is indicated to reduce the need for allogeneic RBC transfusions among patients with perioperative hemoglobin > 10 to ≤ 13 g/dL who are at high risk for perioperative blood loss from elective, noncardiac, nonvascular surgery. PROCRIT is not indicated for patients who are willing to donate autologous blood pre-operatively.
1.5 Limitations of Use
PROCRIT has not been shown to improve quality of life, fatigue, or patient well-being.
PROCRIT is not indicated for use:
- In patients with cancer receiving hormonal agents, biologic products, or radiotherapy, unless also receiving concomitant myelosuppressive chemotherapy.
- In patients with cancer receiving myelosuppressive chemotherapy when the anticipated outcome is cure.
- In patients with cancer receiving myelosuppressive chemotherapy in whom the anemia can be managed by transfusion.
- In patients scheduled for surgery who are willing to donate autologous blood.
- In patients undergoing cardiac or vascular surgery.
- As a substitute for RBC transfusions in patients who require immediate correction of anemia.
2. Procrit Dosage and Administration
2.2 Patients with Chronic Kidney Disease
In controlled trials, patients experienced greater risks for death, serious adverse cardiovascular reactions, and stroke when administered erythropoiesis-stimulating agents (ESAs) to target a hemoglobin level of greater than 11 g/dL. No trial has identified a hemoglobin target level, ESA dose, or dosing strategy that does not increase these risks. Individualize dosing and use the lowest dose of PROCRIT sufficient to reduce the need for RBC transfusions [see Warnings and Precautions (5.1)]. Physicians and patients should weigh the possible benefits of decreasing transfusions against the increased risks of death and other serious cardiovascular adverse reactions [see Boxed Warning and Clinical Studies (14)].
2.4 Patients on Cancer Chemotherapy
Initiate PROCRIT in patients on cancer chemotherapy only if the hemoglobin is less than 10 g/dL, and if there is a minimum of two additional months of planned chemotherapy.
Use the lowest dose of PROCRIT necessary to avoid RBC transfusions.
2.5 Surgery Patients
The recommended PROCRIT regimens are:
- 300 Units/kg per day subcutaneously for 15 days total: administered daily for 10 days before surgery, on the day of surgery, and for 4 days after surgery.
- 600 Units/kg subcutaneously in 4 doses administered 21, 14, and 7 days before surgery and on the day of surgery.
Deep venous thrombosis prophylaxis is recommended during PROCRIT therapy [see Warnings and Precautions (5.1)].
2.6 Preparation and Administration
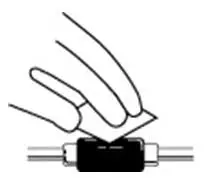
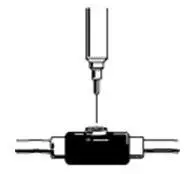
- Do not shake. Do not use PROCRIT that has been shaken or frozen.
- Protect vials from light.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. Do not use any vials exhibiting particulate matter or discoloration.
- Discard unused portions of PROCRIT in preservative-free vials. Do not re-enter preservative-free vials.
- Store unused portions of PROCRIT in multiple-dose vials at 36°F to 46°F (2°C to 8°C). Discard 21 days after initial entry.
- Do not dilute. Do not mix with other drug solutions except for admixing as described below:
Preservative-free PROCRIT from single-dose vials may be admixed in a syringe with bacteriostatic 0.9% sodium chloride injection, USP, with benzyl alcohol 0.9% (bacteriostatic saline) in a 1:1 ratio using aseptic technique at the time of administration. Do not mix PROCRIT with bacteriostatic saline when administering to pregnant women, lactating women, neonates, and infants [see Use in Specific Populations (8.1, 8.2, 8.4)].
3. Dosage Forms and Strengths
Injection:
- 2,000 Units/mL, 3,000 Units/mL, 4,000 Units/mL, 10,000 Units/mL, and 40,000 Units/mL of PROCRIT as a clear and colorless liquid in single-dose vials
- 20,000 Units/2 mL (10,000 Units/mL) and 20,000 Units/mL of PROCRIT as a clear and colorless liquid in multiple-dose vials (contains benzyl alcohol)
4. Contraindications
PROCRIT is contraindicated in patients with:
- Uncontrolled hypertension [see Warnings and Precautions (5.3)]
- Pure red cell aplasia (PRCA) that begins after treatment with PROCRIT or other erythropoietin protein drugs [see Warnings and Precautions (5.6)]
- Serious allergic reactions to PROCRIT [see Warnings and Precautions (5.7)]
PROCRIT from multiple-dose vials contains benzyl alcohol and is contraindicated in:
- Neonates, infants, pregnant women, and lactating women [see Warnings and Precautions (5.9), Use in Specific Populations (8.1, 8.2, 8.4)].
5. Warnings and Precautions
5.1 Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism
- In controlled clinical trials of patients with CKD comparing higher hemoglobin targets (13 – 14 g/dL) to lower targets (9 – 11.3 g/dL), PROCRIT and other ESAs increased the risk of death, myocardial infarction, stroke, congestive heart failure, thrombosis of hemodialysis vascular access, and other thromboembolic events in the higher target groups.
- Using ESAs to target a hemoglobin level of greater than 11 g/dL increases the risk of serious adverse cardiovascular reactions and has not been shown to provide additional benefit [see Clinical Studies (14.1)]. Use caution in patients with coexistent cardiovascular disease and stroke [see Dosage and Administration (2.2)]. Patients with CKD and an insufficient hemoglobin response to ESA therapy may be at even greater risk for cardiovascular reactions and mortality than other patients. A rate of hemoglobin rise of greater than 1 g/dL over 2 weeks may contribute to these risks.
- In controlled clinical trials of patients with cancer, PROCRIT and other ESAs increased the risks for death and serious adverse cardiovascular reactions. These adverse reactions included myocardial infarction and stroke.
- In controlled clinical trials, ESAs increased the risk of death in patients undergoing coronary artery bypass graft surgery (CABG) and the risk of deep venous thrombosis (DVT) in patients undergoing orthopedic procedures.
The design and overall results of the 3 large trials comparing higher and lower hemoglobin targets are shown in Table 1.
| Normal Hematocrit Study (NHS) (N=1265) | CHOIR (N=1432) | TREAT (N=4038) |
|
|---|---|---|---|
| Time Period of Trial | 1993 to 1996 | 2003 to 2006 | 2004 to 2009 |
| Population | CKD patients on hemodialysis with coexisting CHF or CAD, hematocrit 30 ± 3% on epoetin alfa | CKD patients not on dialysis with hemoglobin < 11 g/dL not previously administered epoetin alfa | CKD patients not on dialysis with type II diabetes, hemoglobin ≤ 11 g/dL |
| Hemoglobin Target; Higher vs. Lower (g/dL) | 14.0 vs. 10.0 | 13.5 vs. 11.3 | 13.0 vs. ≥ 9.0 |
| Median (Q1, Q3) Achieved Hemoglobin level (g/dL) | 12.6 (11.6, 13.3) vs. 10.3 (10.0, 10.7) | 13.0 (12.2, 13.4) vs. 11.4 (11.1, 11.6) | 12.5 (12.0, 12.8) vs. 10.6 (9.9, 11.3) |
| Primary Endpoint | All-cause mortality or non-fatal MI | All-cause mortality, MI, hospitalization for CHF, or stroke | All-cause mortality, MI, myocardial ischemia, heart failure, and stroke |
| Hazard Ratio or Relative Risk (95% CI) | 1.28 (1.06 – 1.56) | 1.34 (1.03 – 1.74) | 1.05 (0.94 – 1.17) |
| Adverse Outcome for Higher Target Group | All-cause mortality | All-cause mortality | Stroke |
| Hazard Ratio or Relative Risk (95% CI) | 1.27 (1.04 – 1.54) | 1.48 (0.97 – 2.27) | 1.92 (1.38 – 2.68) |
5.2 Increased Mortality and/or Increased Risk of Tumor Progression or Recurrence in Patients With Cancer
ESAs resulted in decreased locoregional control/progression-free survival (PFS) and/or overall survival (OS) (see Table 2).
Adverse effects on PFS and/or OS were observed in studies of patients receiving chemotherapy for breast cancer (Studies 1, 2, and 4), lymphoid malignancy (Study 3), and cervical cancer (Study 5); in patients with advanced head and neck cancer receiving radiation therapy (Studies 6 and 7); and in patients with non-small cell lung cancer or various malignancies who were not receiving chemotherapy or radiotherapy (Studies 8 and 9).
| Study/Tumor/(n) | Hemoglobin Target | Achieved Hemoglobin (Median; Q1, Q3*) | Primary Efficacy Outcome | Adverse Outcome for ESA- containing Arm |
|---|---|---|---|---|
|
||||
| Chemotherapy | ||||
| Study 1
Metastatic breast cancer (n=2098) | ≤12 g/dL† | 11.6 g/dL 10.7, 12.1 g/dL | Progression-free survival (PFS) | Decreased progression-free and overall survival |
| Study 2
Metastatic breast cancer (n=939) | 12–14 g/dL | 12.9 g/dL; 12.2, 13.3 g/dL | 12-month overall survival | Decreased 12-month survival |
| Study 3
Lymphoid malignancy (n=344) | 13–15 g/dL (M) 13–14 g/dL (F) | 11 g/dL; 9.8, 12.1 g/dL | Proportion of patients achieving a hemoglobin response | Decreased overall survival |
| Study 4
Early breast cancer (n=733) | 12.5–13 g/dL | 13.1 g/dL; 12.5, 13.7 g/dL | Relapse-free and overall survival | Decreased 3-year relapse-free and overall survival |
| Study 5
Cervical cancer (n=114) | 12–14 g/dL | 12.7 g/dL; 12.1, 13.3 g/dL | Progression-free and overall survival and locoregional control | Decreased 3-year progression-free and overall survival and locoregional control |
| Radiotherapy Alone | ||||
| Study 6
Head and neck cancer (n=351) | ≥ 15 g/dL (M) ≥ 14 g/dL (F) | Not available | Locoregional progression-free survival | Decreased 5-year locoregional progression-free and overall survival |
| Study 7
Head and neck cancer (n=522) | 14–15.5 g/dL | Not available | Locoregional disease control | Decreased locoregional disease control |
| No Chemotherapy or Radiotherapy | ||||
| Study 8
Non-small cell lung cancer (n=70) | 12–14 g/dL | Not available | Quality of life | Decreased overall survival |
| Study 9
Non-myeloid malignancy (n=989) | 12–13 g/dL | 10.6 g/dL; 9.4, 11.8 g/dL | RBC transfusions | Decreased overall survival |
5.3 Hypertension
PROCRIT is contraindicated in patients with uncontrolled hypertension. Following initiation and titration of PROCRIT, approximately 25% of patients on dialysis required initiation of or increases in antihypertensive therapy; hypertensive encephalopathy and seizures have been reported in patients with CKD receiving PROCRIT.
Appropriately control hypertension prior to initiation of and during treatment with PROCRIT. Reduce or withhold PROCRIT if blood pressure becomes difficult to control. Advise patients of the importance of compliance with antihypertensive therapy and dietary restrictions [see Patient Counseling Information (17)].
5.4 Seizures
PROCRIT increases the risk of seizures in patients with CKD. During the first several months following initiation of PROCRIT, monitor patients closely for premonitory neurologic symptoms. Advise patients to contact their healthcare practitioner for new-onset seizures, premonitory symptoms or change in seizure frequency.
5.5 Lack or Loss of Hemoglobin Response to PROCRIT
For lack or loss of hemoglobin response to PROCRIT, initiate a search for causative factors (e.g., iron deficiency, infection, inflammation, bleeding). If typical causes of lack or loss of hemoglobin response are excluded, evaluate for PRCA [see Warnings and Precautions (5.6)]. In the absence of PRCA, follow dosing recommendations for management of patients with an insufficient hemoglobin response to PROCRIT therapy [see Dosage and Administration (2.2)].
5.6 Pure Red Cell Aplasia
Cases of PRCA and of severe anemia, with or without other cytopenias that arise following the development of neutralizing antibodies to erythropoietin have been reported in patients treated with PROCRIT. This has been reported predominantly in patients with CKD receiving ESAs by subcutaneous administration. PRCA has also been reported in patients receiving ESAs for anemia related to hepatitis C treatment (an indication for which PROCRIT is not approved).
If severe anemia and low reticulocyte count develop during treatment with PROCRIT, withhold PROCRIT and evaluate patients for neutralizing antibodies to erythropoietin. Contact Janssen Products, LP at 1-800-JANSSEN (1-800-526-7736) to perform assays for binding and neutralizing antibodies. Permanently discontinue PROCRIT in patients who develop PRCA following treatment with PROCRIT or other erythropoietin protein drugs. Do not switch patients to other ESAs.
5.7 Serious Allergic Reactions
Serious allergic reactions, including anaphylactic reactions, angioedema, bronchospasm, skin rash, and urticaria may occur with PROCRIT. Immediately and permanently discontinue PROCRIT and administer appropriate therapy if a serious allergic or anaphylactic reaction occurs.
5.8 Severe Cutaneous Reactions
Blistering and skin exfoliation reactions including Erythema multiforme and Stevens-Johnson Syndrome (SJS)/Toxic Epidermal Necrolysis (TEN), have been reported in patients treated with ESAs (including PROCRIT) in the postmarketing setting. Discontinue PROCRIT therapy immediately if a severe cutaneous reaction, such as SJS/TEN, is suspected.
5.9 Risk of Serious Adverse Reactions Due to Benzyl Alcohol Preservative
PROCRIT from multiple-dose vials contains benzyl alcohol and is contraindicated for use in neonates, infants, pregnant women, and lactating women [see Contraindications (4)]. In addition, do not mix PROCRIT with bacteriostatic saline (which also contains benzyl alcohol) when administering PROCRIT to these patient populations [see Dosage and Administration (2)].
Serious and fatal reactions including "gasping syndrome" can occur in neonates and infants treated with benzyl alcohol-preserved drugs, including PROCRIT multiple-dose vials. The "gasping syndrome" is characterized by central nervous system depression, metabolic acidosis, and gasping respirations. There is a potential for similar risks to fetuses and infants exposed to benzyl alcohol in utero or in breast-fed milk, respectively. PROCRIT multiple-dose vials contain 11 mg of benzyl alcohol per mL. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known [see Use in Specific Populations (8.1, 8.2, and 8.4)].
6. Adverse Reactions/Side Effects
The following serious adverse reactions are discussed in greater detail in other sections of the label:
- Increased Mortality, Myocardial Infarction, Stroke, and Thromboembolism [see Warnings and Precautions (5.1)]
- Increased mortality and/or increased risk of tumor progression or recurrence in Patients With Cancer [see Warnings and Precautions (5.2)]
- Hypertension [see Warnings and Precautions (5.3)]
- Seizures [see Warnings and Precautions (5.4)]
- PRCA [see Warnings and Precautions (5.6)]
- Serious allergic reactions [see Warnings and Precautions (5.7)]
- Severe Cutaneous Reactions [see Warnings and Precautions (5.8)]
6.1 Clinical Trial Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of other drugs and may not reflect the rates observed in practice.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of PROCRIT.
Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Seizures [see Warnings and Precautions (5.4)]
- PRCA [see Warnings and Precautions (5.6)]
- Serious allergic reactions [see Warnings and Precautions (5.7)]
- Injection site reactions, including irritation and pain
- Porphyria
- Severe Cutaneous Reactions [see Warnings and Precautions (5.8)]
6.3 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to epoetin alfa with the incidence of antibodies to other products may be misleading.
Neutralizing antibodies to epoetin alfa that cross-react with endogenous erythropoietin and other ESAs can result in PRCA or severe anemia (with or without other cytopenias) [see Warnings and Precautions (5.6)].
8. Use In Specific Populations
8.4 Pediatric Use
The multiple-dose vials are formulated with benzyl alcohol and are contraindicated for use in neonates and infants [see Contraindications (4), Warnings and Precautions (5.9)]. When therapy with PROCRIT is needed in neonates and infants, use the single-dose vial, which is a benzyl alcohol-free formulation. Do not mix the single-dose vials with bacteriostatic saline when administering PROCRIT to neonates or infants because it contains benzyl alcohol [see Dosage and Administration (2.6)].
Serious adverse reactions including fatal reactions and the "gasping syndrome" occurred in premature neonates and infants in the neonatal intensive care unit who received drugs containing benzyl alcohol as a preservative. In these cases, benzyl alcohol dosages of 99 to 234 mg/kg/day produced high levels of benzyl alcohol and its metabolites in the blood and urine (blood levels of benzyl alcohol were 0.61 to 1.378 mmol/L). Additional adverse reactions included gradual neurological deterioration, seizures, intracranial hemorrhage, hematologic abnormalities, skin breakdown, hepatic and renal failure, hypotension, bradycardia, and cardiovascular collapse. Preterm, low birth weight infants may be more likely to develop these reactions because they may be less able to metabolize benzyl alcohol. The minimum amount of benzyl alcohol at which serious adverse reactions may occur is not known [see Warnings and Precautions (5.9)].
8.5 Geriatric Use
Of the 4553 patients who received PROCRIT in the 6 studies for treatment of anemia due to CKD not receiving dialysis, 2726 (60%) were age 65 years and over, while 1418 (31%) were 75 years and over. Of the 757 patients who received PROCRIT in the 3 studies of CKD patients on dialysis, 361 (47%) were age 65 years and over, while 100 (13%) were 75 years and over. No differences in safety or effectiveness were observed between geriatric and younger patients. Dose selection and adjustment for an elderly patient should be individualized to achieve and maintain the target hemoglobin [see Dosage and Administration (2)].
Among 778 patients enrolled in the 3 clinical studies of PROCRIT for the treatment of anemia due to concomitant chemotherapy, 419 received PROCRIT and 359 received placebo. Of the 419 who received PROCRIT, 247 (59%) were age 65 years and over, while 78 (19%) were 75 years and over. No overall differences in safety or effectiveness were observed between geriatric and younger patients. The dose requirements for PROCRIT in geriatric and younger patients within the 3 studies were similar.
Among 1731 patients enrolled in the 6 clinical studies of PROCRIT for reduction of allogeneic RBC transfusions in patients undergoing elective surgery, 1085 received PROCRIT and 646 received placebo or standard of care treatment. Of the 1085 patients who received PROCRIT, 582 (54%) were age 65 years and over, while 245 (23%) were 75 years and over. No overall differences in safety or effectiveness were observed between geriatric and younger patients. The dose requirements for PROCRIT in geriatric and younger patients within the 4 studies using the 3 times weekly schedule and 2 studies using the weekly schedule were similar.
Insufficient numbers of patients age 65 years or older were enrolled in clinical studies of PROCRIT for the treatment of patients treated with zidovudine for HIV-infection to determine whether they respond differently from younger patients.
10. Overdosage
PROCRIT overdosage can cause hemoglobin levels above the desired level, which should be managed with discontinuation or reduction of PROCRIT dosage and/or with phlebotomy, as clinically indicated [see Pharmacodynamics (12.2)]. Cases of severe hypertension have been observed following overdose with ESAs [see Warnings and Precautions (5.3)].
11. Procrit Description
Epoetin alfa is a 165-amino acid erythropoiesis-stimulating glycoprotein manufactured by recombinant DNA technology. It has a molecular weight of approximately 30,400 daltons and is produced by mammalian cells into which the human erythropoietin gene has been introduced. The product contains the identical amino acid sequence of isolated natural erythropoietin.
PROCRIT (epoetin alfa) injection for intravenous or subcutaneous administration is formulated as a sterile, clear, colorless liquid in vials in multiple formulations. Single-dose vials, formulated with an isotonic sodium chloride/sodium citrate-buffered solution, are supplied in multiple strengths. Each single-dose 1 mL vial contains 2,000, 3,000, 4,000, or 10,000 Units of epoetin alfa, Albumin (Human) (2.5 mg), citric acid (0.06 mg), sodium chloride (5.9 mg), and sodium citrate (5.8 mg) in Water for Injection, USP (pH 6.9 ± 0.3). Single-dose 1 mL vials formulated with an isotonic sodium chloride/sodium phosphate buffer contain 40,000 Units of epoetin alfa albumin (human) (2.5 mg), citric acid (0.0068 mg), sodium chloride (5.8 mg), sodium citrate (0.7 mg), sodium phosphate dibasic anhydrate (1.8 mg), and sodium phosphate monobasic monohydrate (1.2 mg) in Water for Injection, USP (pH 6.9 ± 0.3). Multiple-dose, 2 mL vials contain 10,000 Units epoetin alfa, albumin (human) (2.5 mg), benzyl alcohol (1%), sodium chloride (8.2 mg), citric acid (0.11 mg), and sodium citrate (1.3 mg) per 1 mL Water for Injection, USP (pH 6.1 ± 0.3). Multiple-dose 1 mL vials contain 20,000 Units epoetin alfa, albumin (human) (2.5 mg), benzyl alcohol (1%), sodium chloride (8.2 mg), citric acid (0.11 mg), and sodium citrate (1.3 mg), per 1 mL in Water for Injection, USP (pH 6.1 ± 0.3).
12. Procrit - Clinical Pharmacology
12.1 Mechanism of Action
PROCRIT stimulates erythropoiesis by the same mechanism as endogenous erythropoietin.
12.2 Pharmacodynamics
PROCRIT increases the reticulocyte count within 10 days of initiation, followed by increases in the RBC count, hemoglobin, and hematocrit, usually within 2 to 6 weeks. The rate of hemoglobin increase varies among patients and is dependent upon the dose of PROCRIT administered. For correction of anemia in hemodialysis patients, a greater biologic response is not observed at doses exceeding 300 Units/kg 3 times weekly.
12.3 Pharmacokinetics
In adult and pediatric patients with CKD, the elimination half-life (t1/2) of plasma erythropoietin after intravenous administration of PROCRIT ranged from 4 to 13 hours. After subcutaneous administration, Cmax was achieved within 5 to 24 hours. The t1/2 in adult patients with serum creatinine greater than 3 mg/dL was similar between those not on dialysis and those maintained on dialysis. The pharmacokinetic data indicate no apparent difference in PROCRIT t1/2 among adult patients above or below 65 years of age.
A pharmacokinetic study comparing 150 Units/kg subcutaneous 3 times weekly to 40,000 Units subcutaneous weekly dosing regimen was conducted for 4 weeks in healthy subjects (n=12) and for 6 weeks in anemic cancer patients (n=32) receiving cyclic chemotherapy. There was no accumulation of serum erythropoietin after the 2 dosing regimens during the study period. The 40,000 Units weekly regimen had a higher Cmax (3- to 7-fold), longer Tmax (2- to 3-fold), higher AUC0–168 h (2- to 3-fold) of erythropoietin and lower clearance (CL) (50%) than the 150 Units/kg 3 times weekly regimen. In anemic cancer patients, the average t1/2 was similar (40 hours with range of 16 to 67 hours) after both dosing regimens. After the 150 Units/kg 3 times weekly dosing, the values of Tmax and CL were similar (13.3 ± 12.4 vs. 14.2 ± 6.7 hours, and 20.2 ± 15.9 vs. 23.6 ± 9.5 mL/hr/kg) between week 1 when patients were receiving chemotherapy (n=14) and week 3 when patients were not receiving chemotherapy (n=4). Differences were observed after the 40,000 Units weekly dosing with longer Tmax (38 ± 18 hours) and lower CL (9.2 ± 4.7 mL/hr/kg) during week 1 when patients were receiving chemotherapy (n=18) compared with those (22 ± 4.5 hours, 13.9 ± 7.6 mL/hr/kg, respectively) during week 3 when patients were not receiving chemotherapy (n=7).
The pharmacokinetic profile of PROCRIT in pediatric patients appeared similar to that of adults.
The pharmacokinetics of PROCRIT has not been studied in patients with HIV-infection.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
The carcinogenic potential of PROCRIT has not been evaluated.
PROCRIT was not mutagenic or clastogenic under the conditions tested: PROCRIT was negative in the in vitro bacterial reverse mutation assay (Ames test), in the in vitro mammalian cell gene mutation assay (the hypoxanthine-guanine phosphoribosyl transferase [HGPRT] locus), in an in vitro chromosomal aberration assay in mammalian cells, and in the in vivo mouse micronucleus assay.
When administered intravenously to male and female rats prior to and during mating, and to females through the beginning of implantation (up to gestational day 7; dosing stopped prior to the beginning of organogenesis), doses of 100 and 500 Units/kg/day of PROCRIT caused slight increases in pre-implantation loss, post-implantation loss and decreases in the incidence of live fetuses. It is not clear whether these effects reflect a drug effect on the uterine environment or on the conceptus. This animal dose level of 100 Units/kg/day approximates the clinical recommended starting dose, depending on the patient's treatment indication, but may be lower than the clinical dose in patients whose doses have been adjusted.
14. Clinical Studies
14.1 Patients With Chronic Kidney Disease
14.2 Zidovudine-treated Patients With HIV-infection
The safety and efficacy of PROCRIT were evaluated in 4 placebo-controlled studies enrolling 297 anemic patients (hemoglobin < 10 g/dL) with HIV-infection receiving concomitant therapy with zidovudine. In the subgroup of patients (89/125 PROCRIT and 88/130 placebo) with pre-study endogenous serum erythropoietin levels ≤ 500 mUnits/mL, PROCRIT reduced the mean cumulative number of units of blood transfused per patient by approximately 40% as compared to the placebo group. Among those patients who required RBC transfusions at baseline, 43% of patients treated with PROCRIT versus 18% of placebo-treated patients were RBC transfusion-independent during the second and third months of therapy. PROCRIT therapy also resulted in significant increases in hemoglobin in comparison to placebo. When examining the results according to the weekly dose of zidovudine received during month 3 of therapy, there was a statistically significant reduction (p < 0.003) in RBC transfusion requirements in patients treated with PROCRIT (n=51) compared to placebo-treated patients (n=54) whose mean weekly zidovudine dose was ≤ 4200 mg/week.
Approximately 17% of the patients with endogenous serum erythropoietin levels ≤ 500 mUnits/mL receiving PROCRIT in doses from 100 to 200 Units/kg 3 times weekly achieved a hemoglobin of 12.7 g/dL without administration of RBC transfusions or significant reduction in zidovudine dose. In the subgroup of patients whose pre-study endogenous serum erythropoietin levels were > 500 mUnits/mL, PROCRIT therapy did not reduce RBC transfusion requirements or increase hemoglobin compared to the corresponding responses in placebo-treated patients.
14.3 Patients with Cancer on Chemotherapy
The safety and effectiveness of PROCRIT was assessed in two multicenter, randomized (1:1), placebo-controlled, double-blind studies (Study C1 and Study C2) and a pooled analysis of six additional randomized (1:1), multicenter, placebo-controlled, double-blind studies. All studies were conducted in patients with anemia due to concomitantly administered cancer chemotherapy. Study C1 enrolled 344 adult patients, Study C2 enrolled 222 pediatric patients, and the pooled analysis contained 131 patients randomized to epoetin alfa or placebo. In Studies C1 and C2, efficacy was demonstrated by a reduction in the proportion of patients who received an RBC transfusion, from week 5 through end of the study, with the last-known RBC transfusion status carried forward for patients who discontinued treatment. In the pooled analysis, efficacy was demonstrated by a reduction in the proportion of patients who received an RBC transfusion from week 5 through end of the study in the subset of patients who were remaining on therapy for 6 or more weeks.
14.4 Surgery Patients
The safety and efficacy of PROCRIT were evaluated in a placebo-controlled, double-blind study (S1) enrolling 316 patients scheduled for major, elective orthopedic hip or knee surgery who were expected to require ≥ 2 units of blood and who were not able or willing to participate in an autologous blood donation program. Patients were stratified into 1 of 3 groups based on their pretreatment hemoglobin [≤ 10 g/dL (n=2), > 10 to ≤ 13 g/dL (n=96), and > 13 to ≤ 15 g/dL (n=218)] and then randomly assigned to receive 300 Units/kg PROCRIT, 100 Units/kg PROCRIT, or placebo by subcutaneous injection for 10 days before surgery, on the day of surgery, and for 4 days after surgery. All patients received oral iron and a low-dose, postoperative warfarin regimen.
Treatment with PROCRIT 300 Units/kg significantly (p=0.024) reduced the risk of allogeneic RBC transfusion in patients with a pretreatment hemoglobin of > 10 to ≤ 13 g/dL; 5/31 (16%) of patients treated with PROCRIT 300 Units/kg, 6/26 (23%) of patients treated with PROCRIT 100 Units/kg, and 13/29 (45%) of placebo-treated patients were transfused. There was no significant difference in the number of patients transfused between PROCRIT (9% 300 Units/kg, 6% 100 Units/kg) and placebo (13%) in the > 13 to ≤ 15 g/dL hemoglobin stratum. There were too few patients in the ≤ 10 g/dL group to determine if PROCRIT is useful in this hemoglobin strata. In the > 10 to ≤ 13 g/dL pretreatment stratum, the mean number of units transfused per PROCRIT-treated patient (0.45 units blood for 300 Units/kg, 0.42 units blood for 100 Units/kg) was less than the mean transfused per placebo-treated patient (1.14 units) (overall p=0.028). In addition, mean hemoglobin, hematocrit, and reticulocyte counts increased significantly during the presurgery period in patients treated with PROCRIT.
PROCRIT was also evaluated in an open-label, parallel-group study (S2) enrolling 145 patients with a pretreatment hemoglobin level of ≥ 10 to ≤ 13 g/dL who were scheduled for major orthopedic hip or knee surgery and who were not participating in an autologous program. Patients were randomly assigned to receive 1 of 2 subcutaneous dosing regimens of PROCRIT (600 Units/kg once weekly for 3 weeks prior to surgery and on the day of surgery, or 300 Units/kg once daily for 10 days prior to surgery, on the day of surgery, and for 4 days after surgery). All patients received oral iron and appropriate pharmacologic anticoagulation therapy.
From pretreatment to presurgery, the mean increase in hemoglobin in the 600 Units/kg weekly group (1.44 g/dL) was greater than that observed in the 300 Units/kg daily group. The mean increase in absolute reticulocyte count was smaller in the weekly group (0.11 × 106/mm3) compared to the daily group (0.17 × 106/mm3). Mean hemoglobin levels were similar for the 2 treatment groups throughout the postsurgical period.
The erythropoietic response observed in both treatment groups resulted in similar RBC transfusion rates [11/69 (16%) in the 600 Units/kg weekly group and 14/71 (20%) in the 300 Units/kg daily group]. The mean number of units transfused per patient was approximately 0.3 units in both treatment groups.
16. How is Procrit supplied
PROCRIT (epoetin alfa) injection is a sterile, clear, and colorless solution available as:
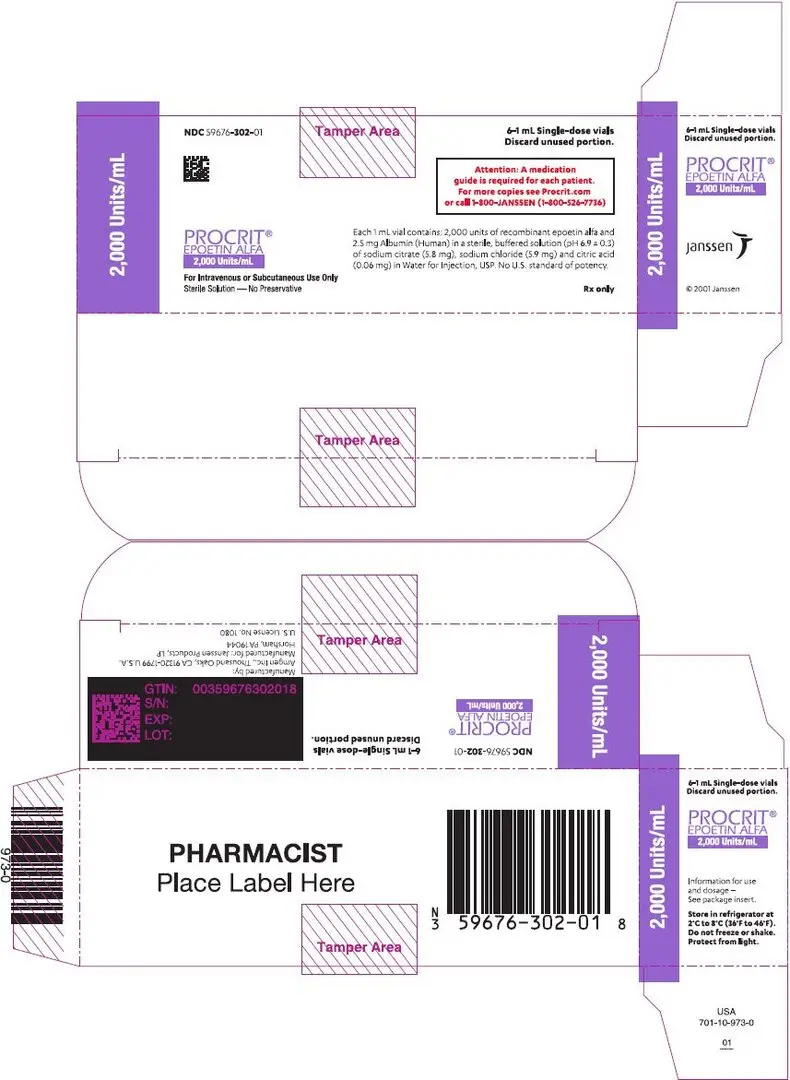
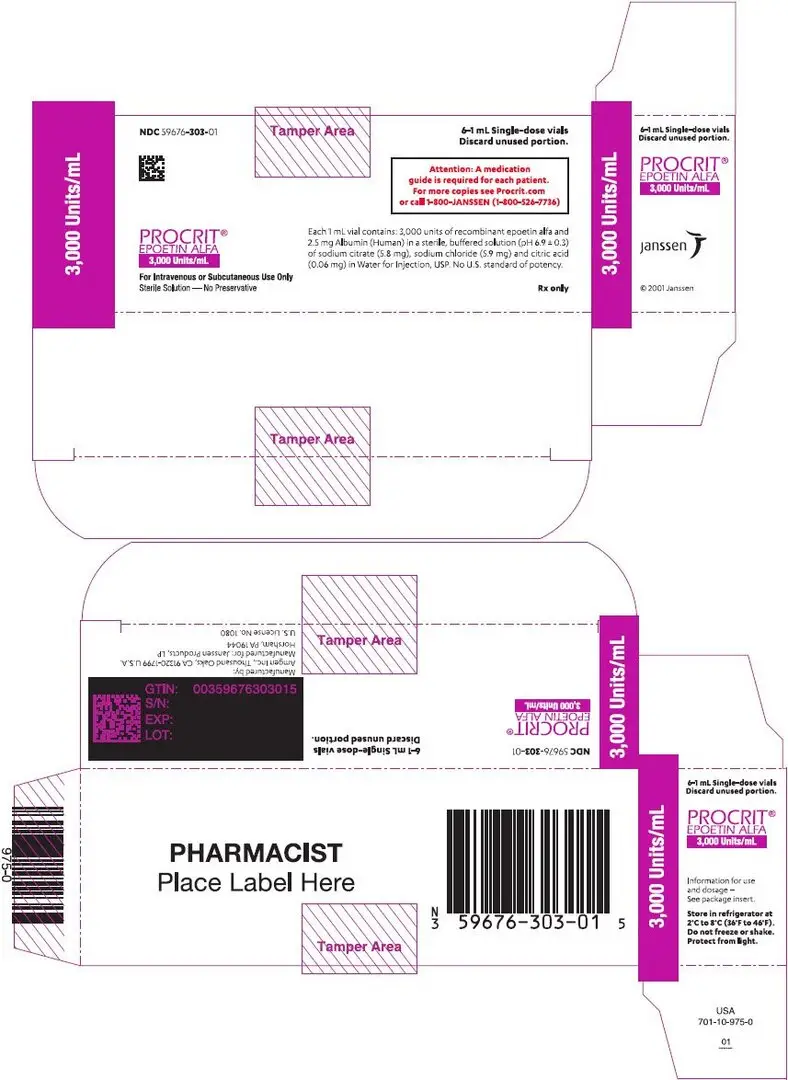
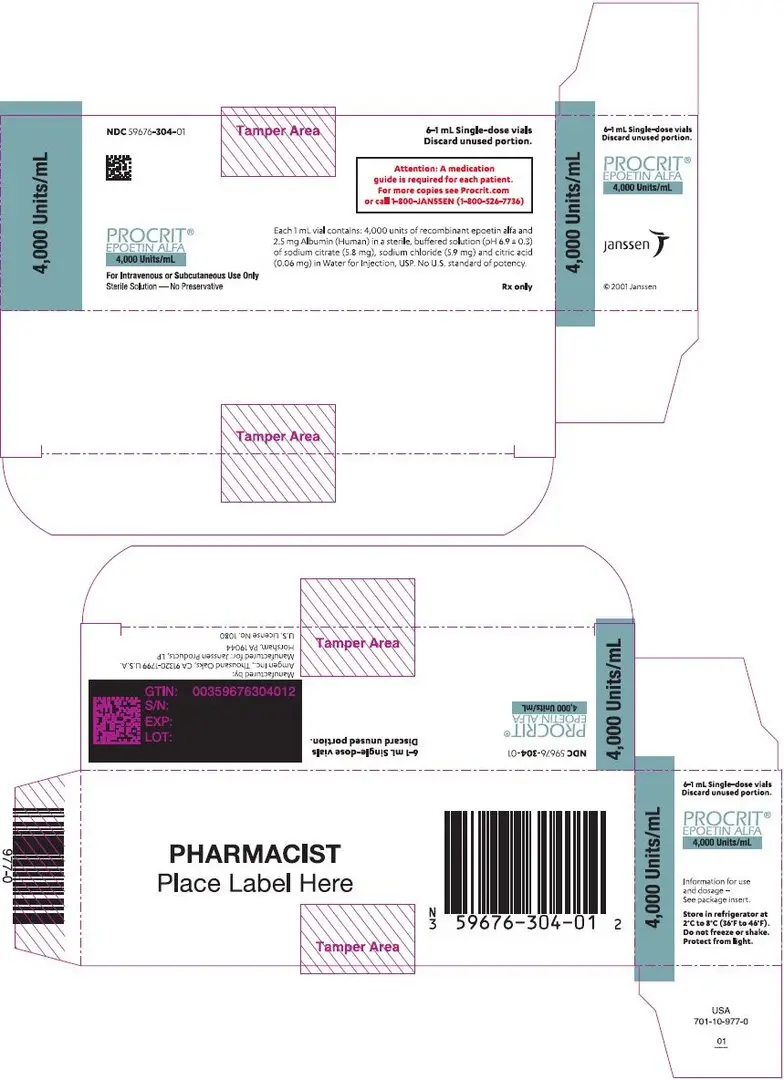
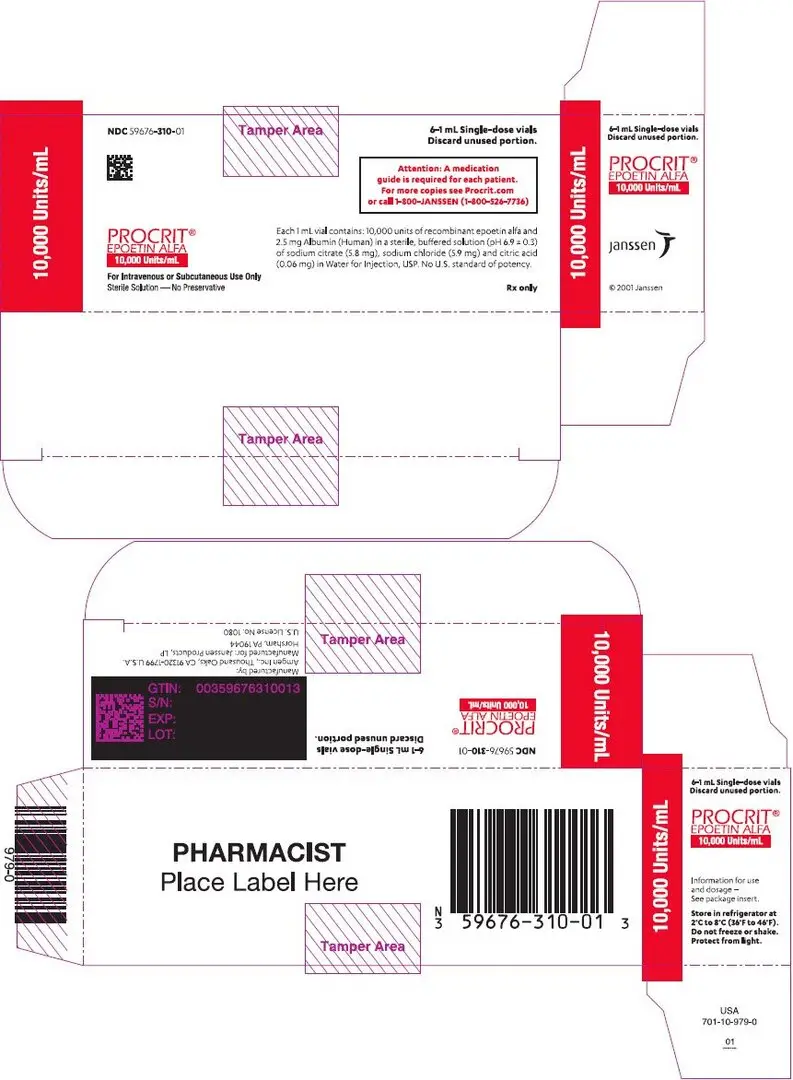
Preservative-free, single-dose vials (in citrate-buffered formulation): 2,000 Units/mL (NDC 59676-302-01), 3,000 Units/mL (NDC 59676-303-01), 4,000 Units/mL (NDC 59676-304-01), or 10,000 Units/mL (NDC 59676-310-01) supplied in cartons, each carton containing six 1 mL single-dose vials.
Preservative-free, single-dose vials (in citrate-buffered formulation): 10,000 Units/mL (NDC 59676-310-02) supplied in dispensing packs (tray) containing 25 single-dose 1 mL vials.
Preservative-free, single-dose vials (in phosphate-buffered formulation): 40,000 Units/mL (NDC 59676-340-01) supplied in dispensing packs containing four 1 mL single-dose vials.
Preserved, multiple-dose vials: 20,000 Units/2mL (10,000 Units/mL) (NDC 59676-312-04) supplied in dispensing packs containing four 2 mL multiple-dose vials.
Preserved, multiple-dose vials: 20,000 Units/mL (NDC 59676-320-04) supplied in dispensing packs containing four 1 mL multiple-dose vials.
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Inform patients:
- Of the increased risks of mortality, serious cardiovascular reactions, thromboembolic reactions, stroke, and tumor progression [see Warnings and Precautions (5.1, 5.2)].
- To undergo regular blood pressure monitoring, adhere to prescribed anti-hypertensive regimen and follow recommended dietary restrictions.
- To contact their healthcare provider for new-onset neurologic symptoms or change in seizure frequency.
- Of the need to have regular laboratory tests for hemoglobin.
- Risks are associated with benzyl alcohol in neonates, infants, pregnant women, and lactating women [see Use in Specific Populations (8.1, 8.2, 8.4)].
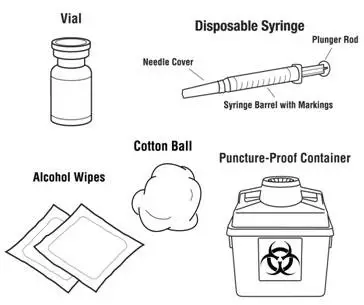
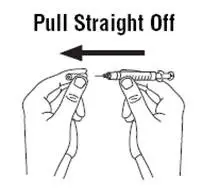
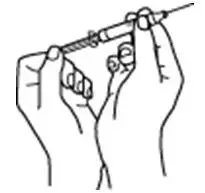
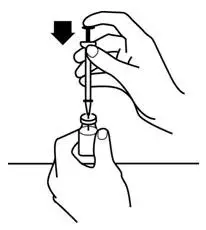
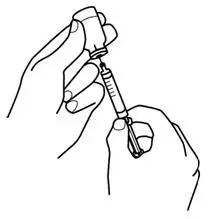
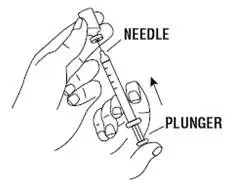
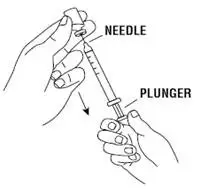
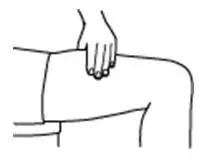
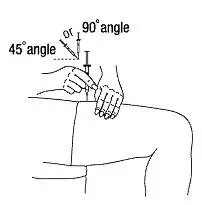
Instruct patients who self-administer PROCRIT of the:
- Importance of following the Instructions for Use.
- Dangers of reusing needles, syringes, or unused portions of single-dose vials.
- Proper disposal of used syringes, needles, and unused vials, and of the full container.
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised 9/2017 | |
| MEDICATION GUIDE PROCRIT® (PRO'–KRIT) (epoetin alfa) |
||
|
Read this Medication Guide:
This Medication Guide does not take the place of talking to your healthcare provider about your medical condition or your treatment. Talk with your healthcare provider regularly about the use of PROCRIT and ask if there is new information about PROCRIT. |
||
|
What is the most important information I should know about PROCRIT? PROCRIT may cause serious side effects that can lead to death, including: For people with cancer:
For all people who take PROCRIT, including people with cancer or chronic kidney disease:
See "What are the possible side effects of PROCRIT?" below for more information. If you decide to take PROCRIT, your healthcare provider should prescribe the smallest dose of PROCRIT that is necessary to reduce your chance of needing RBC transfusions. |
||
|
What is PROCRIT? PROCRIT is a prescription medicine used to treat anemia. People with anemia have a lower-than-normal number of RBCs. PROCRIT works like the human protein called erythropoietin to help your body make more RBCs. PROCRIT is used to reduce or avoid the need for RBC transfusions. PROCRIT may be used to treat anemia if it is caused by:
PROCRIT may also be used to reduce the chance you will need RBC transfusions if you are scheduled for certain surgeries where a lot of blood loss is expected. If your hemoglobin level stays too high or if your hemoglobin goes up too quickly, this may lead to serious health problems which may result in death. These serious health problems may happen if you take PROCRIT, even if you do not have an increase in your hemoglobin level. PROCRIT has not been proven to improve quality of life, fatigue, or well-being. PROCRIT should not be used for treatment of anemia:
PROCRIT should not be used to reduce the chance you will need RBC transfusions if:
It is not known if PROCRIT is safe and effective in treating anemia in children less than 1 month old who have chronic kidney disease and in children less than 5 years old who have anemia caused by chemotherapy. |
||
|
Do not take PROCRIT if you:
Do not give PROCRIT from multiple-dose vials to:
|
||
|
Before taking PROCRIT, tell your healthcare provider about all of your medical conditions, including if you:
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements. |
||
|
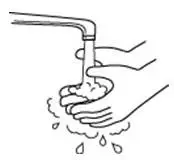
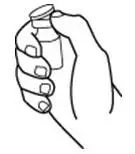
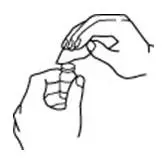
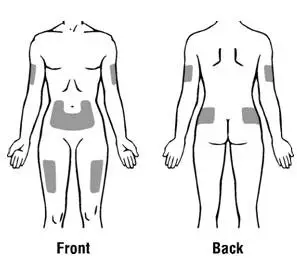
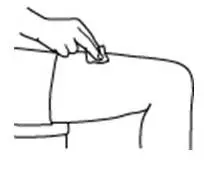
How should I take PROCRIT?
|
||
|
What are the possible side effects of PROCRIT? PROCRIT may cause serious side effects, including:
Common side effects of PROCRIT include: |
||
|
|
|
These are not all of the possible side effects of PROCRIT. Your healthcare provider can give you a more complete list. Tell your healthcare provider about any side effects that bother you or that do not go away. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
||
|
How should I store PROCRIT?
Keep PROCRIT and all medicines out of the reach of children. |
||
|
General information about PROCRIT Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use PROCRIT for a condition for which it was not prescribed. Do not give PROCRIT to other people even if they have the same symptoms that you have. It may harm them. You can ask your healthcare provider or pharmacist for information about PROCRIT that is written for healthcare professionals. |
||
|
What are the ingredients in PROCRIT? Active ingredient: epoetin alfa Inactive ingredients:
Manufactured by:
Manufactured for:
For more information, go to the following website: www.PROCRIT.com or call 1-800-JANSSEN (1-800-526-7736). |
||
| PROCRIT
erythropoietin injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PROCRIT
erythropoietin injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PROCRIT
erythropoietin injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PROCRIT
erythropoietin injection, solution |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| PROCRIT
erythropoietin injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PROCRIT
erythropoietin injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| PROCRIT
erythropoietin injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Janssen Products, LP (804684207) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amgen Manufacturing Ltd | 785800020 | MANUFACTURE(59676-302, 59676-303, 59676-304, 59676-310, 59676-340, 59676-312, 59676-320) , ANALYSIS(59676-302, 59676-303, 59676-304, 59676-310, 59676-340, 59676-312, 59676-320) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Patheon Manufacturing Services LLC | 079415560 | ANALYSIS(59676-302, 59676-303, 59676-304, 59676-310, 59676-340, 59676-312, 59676-320) , MANUFACTURE(59676-302, 59676-303, 59676-304, 59676-310, 59676-340, 59676-312, 59676-320) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Amgen, Inc. | 039976196 | MANUFACTURE(59676-302, 59676-303, 59676-304, 59676-310, 59676-340, 59676-312, 59676-320) , ANALYSIS(59676-302, 59676-303, 59676-304, 59676-310, 59676-340, 59676-312, 59676-320) | |