Drug Detail:Prohance (Gadoteridol [ gad-oh-ter-i-dol ])
Drug Class: Magnetic resonance imaging contrast media
WARNING: NEPHROGENIC SYSTEMIC FIBROSIS
- The risk for NSF appears highest among patients with:
- chronic, severe kidney disease (GFR <30 mL/min/1.73m2), or
- acute kidney injury.
- Screen patients for acute kidney injury and other conditions that may reduce renal function. For patients at risk for chronically reduced renal function (e.g. age > 60 years, hypertension or diabetes), estimate the glomerular filtration rate (GFR) through laboratory testing.
- For patients at highest risk for NSF, do not exceed the recommended ProHance dose and allow a sufficient period of time for elimination of the drug from the body prior to re-administration (see WARNINGS).
ProHance Description
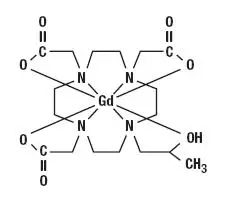
ProHance has a pH of 6.5 to 8.0. Pertinent physicochemical data are noted below:
| PARAMETER | ||
| Osmolality (mOsmol/kg water) | ||
| @ 37° C | 630 | |
| Viscosity | ||
| (cP) | @ 20° C | 2.0 |
| @ 37° C | 1.3 | |
| Specific Gravity | ||
| @ 25° C | 1.140 | |
| Density | ||
| (g/mL) | @ 25° C | 1.137 |
| Octanol: H2O coefficient | -3.68 ± 0.02 | |
ProHance - Clinical Pharmacology
Precautions
General
Personnel trained in resuscitation techniques and resuscitation equipment should be available.
Information for patients:
- is pregnant or breast feeding
- has anemia or diseases that affect the red blood cells
- has a history of renal or hepatic disease, seizure, hemoglobinopathies, asthma or allergic respiratory diseases.
- has recently received a GBCA.
- Describe the clinical manifestations of NSF
- Describe procedures to screen for the detection of renal impairment
Adverse Reactions/Side Effects
The following additional adverse events occurred in fewer than 1% of the patients:
| Body as a Whole: | Facial Edema; Neck Rigidity; Pain; Pain at Injection Site; Injection Site Reaction; Chest Pain; Headache; Fever; Itching; Watery Eyes; Abdominal Cramps; Tingling Sensation in Throat; Laryngismus; Flushed Feeling; Vasovagal Reaction; Anaphylactoid Reactions (characterized by cardiovascular, respiratory and cutaneous symptoms) |
| Cardiovascular: | Prolonged P-R Interval; Hypotension; Elevated Heart Rate; A-V Nodal Rhythm |
| Digestive: | Edematous and/or itching tongue; Gingivitis; Dry Mouth; Loose Bowel; Vomiting |
| Nervous System: | Anxiety; Dizziness; Paresthesia; Mental Status Decline; Loss of Coordination in Arm; Staring Episode; Seizure; Syncope |
| Respiratory System: | Dyspnea; Rhinitis; Cough |
| Skin and Appendages: | Pruritus; Rash; Rash Macular Papular; Urticaria; Hives; Tingling Sensation of Extremity and Digits |
| Special Senses: | Tinnitus |
The following adverse drug reactions have also been reported:
| Body as a Whole: | Generalized Edema; Laryngeal Edema; Malaise; Anaphylactoid Reactions (characterized by cardiovascular, respiratory and cutaneous symptoms, and rarely resulting in Death) |
| Cardiovascular: | Cardiac Arrest; Bradycardia; Hypertension; and Death in association with pre-existing cardiovascular disorders |
| Digestive: | Increased Salivation; Dysphagia |
| Nervous System: | Stupor; Tremor; Loss of Consciousness |
| Respiratory: | Apnea; Wheezing |
| Skin and Appendages: | Sweating; and Cyanosis |
| Special Senses: | Voice Alteration; Transitory Deafness |
| Urogenital: | Urinary Incontinence |
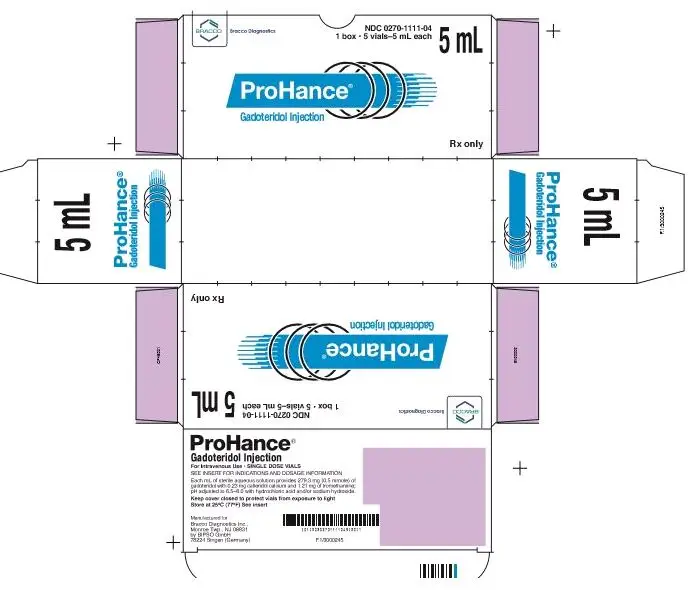
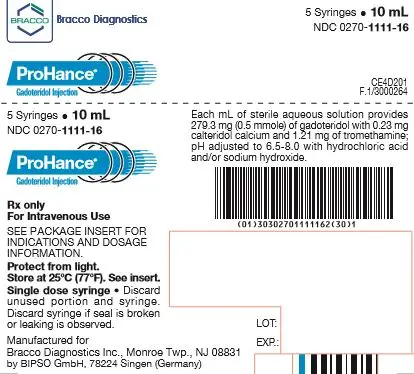
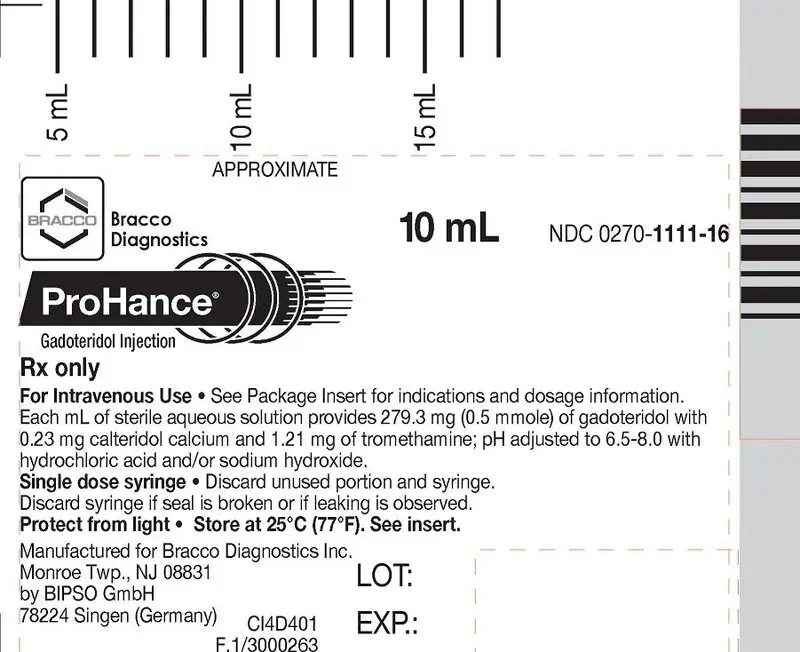
How is ProHance supplied
| Five 5 mL fills in single dose 15 mL vials | (NDC 0270-1111-04) |
| Five 10 mL fills in single dose 30 mL vials | (NDC 0270-1111-01) |
| Five 15 mL fills in single dose 30 mL vials | (NDC 0270-1111-02) |
| Five 20 mL fills in single dose 30 mL vials | (NDC 0270-1111-03) |
| Five 10 mL fills in single dose 20 mL prefilled syringes | (NDC 0270-1111-16) |
| Five 17 mL fills in single dose 20 mL prefilled syringes | (NDC 0270-1111-45) |
Storage and Handling
Directions for Use of the ProHance® (Gadoteridol) Injection single dose syringe*
- 1)
- Screw the threaded tip of the plunger
rod clockwise into the cartridge plunger and push forward a few millimeters
to break any friction between the cartridge plunger and syringe barrel.
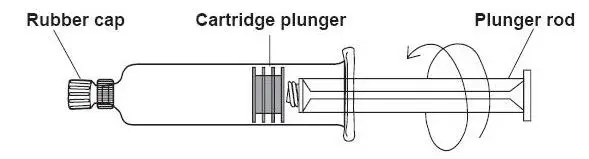
- 2)
- Holding syringe erect, aseptically remove the rubber cap from the tip of the syringe and attach either a sterile, disposable needle or tubing with a compatible luer lock using a push-twist action.
- 3)
- Hold the syringe erect and push plunger forward until all of the air is evacuated and fluid either appears at the tip of the needle or the tubing is filled. Following the usual aspiration procedure, complete the injection. To ensure complete delivery of the contrast medium, the injection should be followed by a normal saline flush.
- 4)
- Properly dispose of the syringe and any other materials used.
*The syringe assembly is a HYPAK SCF® single dose syringe supplied by Becton Dickinson.
This product is covered by one or more of:
U.S. Patent No. 5,474,756; U.S. Patent No. 5,846,519; and U.S. Patent No. 6,143,274.
| PROHANCE
gadoteridol injection, solution |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Labeler - BIPSO GmbH (342104149) |
| Registrant - BIPSO GmbH (342104149) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BIPSO GmbH | 342104149 | MANUFACTURE(76381-111) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Takeda GmbH | 313270016 | ANALYSIS(76381-111) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| BRACCO IMAGING SPA | 434384007 | API MANUFACTURE(76381-111) | |