Drug Detail:Prostin e2 (Dinoprostone topical [ dye-no-pros-tone-top-ik-al ])
Drug Class: Uterotonic agents
Prostin E2 Description
PROSTIN E2 Vaginal Suppository, an oxytocic, contains dinoprostone as the naturally occurring prostaglandin E2 (PGE2).
Its chemical name is (5Z,11α,13E,15S)-11,15-Dihydroxy-9-oxo-prosta-5,13-dien-1-oic acid and the structural formula is represented below:
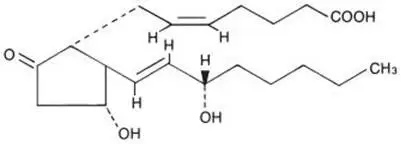
The molecular formula is C20H32O5. The molecular weight of dinoprostone is 352.5. Dinoprostone occurs as a white crystalline powder. It has a melting point within the range of 64° to 71°C. Dinoprostone is soluble in ethanol and in 25% ethanol in water. It is soluble in water to the extent of 130 mg/100 mL.
Each suppository contains 20 mg of dinoprostone in a mixture of glycerides of fatty acids.
Prostin E2 - Clinical Pharmacology
PROSTIN E2 Vaginal Suppository administered intravaginally stimulates the myometrium of the gravid uterus to contract in a manner that is similar to the contractions seen in the term uterus during labor. Whether or not this action results from a direct effect of dinoprostone on the myometrium has not been determined with certainty at this time. Nonetheless, the myometrial contractions induced by the vaginal administration of dinoprostone are sufficient to produce evacuation of the products of conception from the uterus in the majority of cases.
Dinoprostone is also capable of stimulating the smooth muscle of the gastrointestinal tract of man. This activity may be responsible for the vomiting and/or diarrhea that is not uncommon when dinoprostone is used to terminate pregnancy.
In laboratory animals, and also in man, large doses of dinoprostone can lower blood pressure, probably as a consequence of its effect on the smooth muscle of the vascular system. With the doses of dinoprostone used for terminating pregnancy this effect has not been clinically significant. In laboratory animals, and also in man, dinoprostone can elevate body temperature. With the clinical doses of dinoprostone used for the termination of pregnancy some patients do exhibit temperature increases.
Adverse Reactions/Side Effects
The most frequent adverse reactions observed with the use of dinoprostone for abortion are related to its contractile effect on smooth muscle.
In the patients studied, approximately two-thirds experienced vomiting, one-half temperature elevations, two-fifths diarrhea, one-third some nausea, one-tenth headache, and one-tenth shivering and chills.
In addition, approximately one-tenth of the patients studied exhibited transient diastolic blood pressure decreases of greater than 20 mmHg.
Two cases of myocardial infarction following the use of dinoprostone have been reported in patients with a history of cardiovascular disease.
It is not known whether these events were related to the administration of dinoprostone.
Adverse effects in decreasing order of their frequency, observed with the use of dinoprostone, not all of which are clearly drug related include:
| Vomiting Diarrhea Nausea Fever Headache Chills or shivering Backache Joint inflammation or pain new or exacerbated Flushing or hot flashes Dizziness Arthralgia Vaginal pain Chest pain Dyspnea Endometritis Syncope or fainting sensation Vaginitis or vulvitis Weakness Muscular cramp or pain Tightness in chest | Nocturnal leg cramps Uterine rupture Breast tenderness Blurred vision Coughing Rash Myalgia Stiff neck Dehydration Tremor Paresthesia Hearing impairment Urine retention Pharyngitis Laryngitis Diaphoresis Eye pain Wheezing Cardiac arrhythmia Skin discoloration Vaginismus Tension |
| PROSTIN
E2
dinoprostone suppository |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Pharmacia and Upjohn Company LLC (618054084) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Pharmacia and Upjohn Company LLC | 618054084 | MANUFACTURE(0009-0827) | |