Drug Detail:Ravicti (Glycerol phenylbutyrate [ glis-er-ol-fen-il-bue-ti-rate ])
Drug Class: Urea cycle disorder agents
Highlights of Prescribing Information
RAVICTI® (glycerol phenylbutyrate) oral liquid
Initial U.S. Approval: 1996
Recent Major Changes
| Dosage and Administration (2.1, 2.6) | 9/2021 |
Indications and Usage for Ravicti Oral Liquid
RAVICTI is a nitrogen-binding agent indicated for chronic management of patients with urea cycle disorders (UCDs) who cannot be managed by dietary protein restriction and/or amino acid supplementation alone. RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements. (1)
Limitations of Use:
- RAVICTI is not indicated for treatment of acute hyperammonemia in patients with UCDs. (1)
- Safety and efficacy for treatment of N-acetylglutamate synthase (NAGS) deficiency has not been established. (1)
Ravicti Oral Liquid Dosage and Administration
- RAVICTI should be prescribed by a physician experienced in management of UCDs. For administration and preparation, see full prescribing information. (2.1, 2.6)
Switching From Sodium Phenylbutyrate Tablets or Powder to RAVICTI:
- Patients should receive the dosage of RAVICTI that contains the same amount of phenylbutyric acid, see full prescribing information for conversion. (2.2)
Initial Dosage in Phenylbutyrate-Naïve Patients (2.3):
- Recommended dosage range is 4.5 to 11.2 mL/m2/day (5 to 12.4 g/m2/day).
- For patients with some residual enzyme activity not adequately controlled with dietary restriction, the recommended starting dose is 4.5 mL/m2/day.
- Take into account patient's estimated urea synthetic capacity, dietary protein intake, and diet adherence.
Dosage Adjustment and Monitoring:
- Follow plasma ammonia levels to determine the need for dosage titration. (2.4)
Dosage Modifications in Patients with Hepatic Impairment:
- Start dosage at lower end of range. (2.5, 8.7)
Dosage Forms and Strengths
Oral liquid: 1.1 g/mL. (3)
Contraindications
Known hypersensitivity to phenylbutyrate. (4)
Warnings and Precautions
- Neurotoxicity: Phenylacetate (PAA), the active moiety of RAVICTI, may be toxic; reduce dosage for symptoms of neurotoxicity. (5.1)
- Pancreatic Insufficiency or Intestinal Malabsorption: Monitor ammonia levels closely. (5.2)
Adverse Reactions/Side Effects
Most common adverse reactions (≥10%) in adults are: diarrhea, flatulence, and headache. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Horizon at 1-866-479-6742 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Corticosteroids, valproic acid, or haloperidol: May increase plasma ammonia level; monitor ammonia levels closely. (7.1)
- Probenecid: May affect renal excretion of metabolites of RAVICTI, including phenylacetylglutamine (PAGN) and PAA. (7.2)
- CYP3A4 Substrates with narrow therapeutic index (e.g., alfentanil, quinidine, cyclosporine): RAVICTI may decrease exposure; monitor for decreased efficacy of the narrow therapeutic index drug. (7.3)
- Midazolam: Decreased exposure; monitor for suboptimal effect of midazolam. (7.3)
Use In Specific Populations
Lactation: Breastfeeding is not recommended. (8.2)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 9/2021
Related/similar drugs
Buphenyl, sodium phenylbutyrate, Olpruva, Pheburane, glycerol phenylbutyrateFull Prescribing Information
1. Indications and Usage for Ravicti Oral Liquid
RAVICTI is indicated for use as a nitrogen-binding agent for chronic management of patients with urea cycle disorders (UCDs) who cannot be managed by dietary protein restriction and/or amino acid supplementation alone. RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (e.g., essential amino acids, arginine, citrulline, protein-free calorie supplements).
2. Ravicti Oral Liquid Dosage and Administration
2.1 Important Administration Instructions
RAVICTI should be prescribed by a physician experienced in the management of UCDs.
- Instruct patients to take RAVICTI with food or formula and to administer directly into the mouth via oral syringe.
-
Instruct patients to use the RAVICTI bottle and oral syringe as follows:
- Use a new reclosable bottle cap adapter with each new bottle that is opened.
- Open the RAVICTI bottle and twist on the new reclosable bottle cap adapter.
- Use a new and dry oral syringe to withdraw each prescribed dose of RAVICTI.
- Discard the oral syringe after each dose.
- Tightly close the tethered tab on the reclosable bottle cap adapter after each use.
- Do not rinse the reclosable bottle cap adapter.
- Discard bottle and any remaining contents 28 days after opening.
- If water or moisture enters the RAVICTI bottle, the contents will become cloudy in appearance. If the contents of the bottle appear cloudy at any time, do not use the remaining RAVICTI in the bottle and return it to the pharmacy to be discarded.
- Instruct that RAVICTI should be administered just prior to breastfeeding in infants who are breastfeeding.
- For patients who cannot swallow, see the instructions on administration of RAVICTI by nasogastric tube or gastrostomy tube [see Dosage and Administration (2.6)].
- For patients who require a volume of less than 1 mL per dose via nasogastric or gastrostomy tube, the delivered dose may be less than anticipated. Closely monitor these patients using ammonia levels [see Dosage and Administration (2.6)].
- The recommended dosages for patients switching from sodium phenylbutyrate to RAVICTI and patients naïve to phenylbutyric acid are different [see Dosage and Administration (2.2, 2.3)]. For both subpopulations:
- Patients 2 years of age and older: Give RAVICTI in 3 equally divided dosages, each rounded up to the nearest 0.5 mL
- Patients less than 2 years: Give RAVICTI in 3 or more equally divided dosages, each rounded up to the nearest 0.1 mL.
- The maximum total daily dosage is 17.5 mL (19 g).
- RAVICTI must be used with dietary protein restriction and, in some cases, dietary supplements (e.g., essential amino acids, arginine, citrulline, protein-free calorie supplements).
2.2 Switching From Sodium Phenylbutyrate to RAVICTI
Patients switching from sodium phenylbutyrate to RAVICTI should receive the dosage of RAVICTI that contains the same amount of phenylbutyric acid. The conversion is as follows:
Total daily dosage of RAVICTI (mL) = total daily dosage of sodium phenylbutyrate tablets (g) × 0.86
Total daily dosage of RAVICTI (mL) = total daily dosage of sodium phenylbutyrate powder (g) × 0.81
2.3 Initial Dosage in Phenylbutyrate-Naïve Patients
The recommended dosage range, based upon body surface area, in patients naïve to phenylbutyrate (PBA) is 4.5 to 11.2 mL/m2/day (5 to 12.4 g/m2/day). For patients with some residual enzyme activity who are not adequately controlled with protein restriction, the recommended starting dosage is 4.5 mL/m2/day.
In determining the starting dosage of RAVICTI in treatment-naïve patients, consider the patient's residual urea synthetic capacity, dietary protein requirements, and diet adherence. Dietary protein is approximately 16% nitrogen by weight. Given that approximately 47% of dietary nitrogen is excreted as waste and approximately 70% of an administered PBA dose will be converted to urinary phenylacetylglutamine (U-PAGN), an initial estimated RAVICTI dose for a 24-hour period is 0.6 mL RAVICTI per gram of dietary protein ingested per 24-hour period. The total daily dosage should not exceed 17.5 mL.
2.4 Dosage Adjustment and Monitoring
During treatment with RAVICTI, patients should be followed clinically and with plasma ammonia levels to determine the need for dosage titration. Closely monitor plasma ammonia levels during treatment with RAVICTI and when changing the dosage of RAVICTI.
The methods used for measuring plasma ammonia levels vary among individual laboratories and values obtained using different assay methods may not be interchangeable. Normal ranges and therapeutic target levels for plasma ammonia depend upon the assay method used by the individual laboratory. During treatment with RAVICTI, refer to the assay-specific normal ranges and to the therapeutic target ranges for plasma ammonia.
2.5 Dosage Modifications in Patients with Hepatic Impairment
For patients with moderate to severe hepatic impairment, the recommended starting dosage is at the lower end of the recommended dosing range (4.5 mL/m2/day) and the dosage should be kept at the lowest necessary to control the patient's plasma ammonia [see Use in Specific Populations (8.7)].
2.6 Preparation for Nasogastric Tube or Gastrostomy Tube Administration
It is recommended that all patients who can swallow take RAVICTI orally, even those with nasogastric and/or gastrostomy tubes. For patients who cannot swallow, a nasogastric tube or gastrostomy tube may be used to administer RAVICTI as follows:
- Utilize a new dry oral syringe to withdraw each prescribed dosage of RAVICTI from the bottle.
- Place the tip of the syringe into the nasogastric/gastrostomy tube.
- Utilizing the plunger of the syringe, administer RAVICTI into the tube.
- Use a separate syringe to flush the nasogastric/gastrostomy tube. Flush once with 10 mL of water or formula and allow the flush to drain.
- If needed, flush a second time with an additional 10 mL of water or formula to clear the tube.
For patients who require a volume of less than 1 mL per dose via nasogastric or gastrostomy tube, the delivered dosage may be less than anticipated due to adherence of RAVICTI to the plastic tubing. Therefore, these patients should be closely monitored using ammonia levels following initiation of RAVICTI dosing or dosage adjustments.
3. Dosage Forms and Strengths
Oral liquid: colorless to pale yellow, 1.1 g/mL of glycerol phenylbutyrate (delivers 1.02 g/mL of phenylbutyrate).
4. Contraindications
RAVICTI is contraindicated in patients with known hypersensitivity to phenylbutyrate. Signs of hypersensitivity include wheezing, dyspnea, coughing, hypotension, flushing, nausea, and rash.
5. Warnings and Precautions
5.1 Neurotoxicity
Increased exposure to PAA, the major metabolite of RAVICTI, may be associated with neurotoxicity in patients with UCDs. In a study of adult cancer patients, subjects received sodium phenylacetate administered as a 1-hour infusion twice daily at two dose levels of 125 and 150 mg/kg for a 2-week period. Of 18 subjects enrolled, 7 had a history of primary central nervous system tumor. Signs and symptoms of potential PAA neurotoxicity, which were reversible, were reported at plasma PAA concentrations above 500 micrograms/mL and included somnolence, fatigue, lightheadedness, headache, dysgeusia, hypoacusis, disorientation, impaired memory, and exacerbation of preexisting neuropathy. PAA concentrations were not measured when symptoms resolved.
In healthy subjects, after administration of 4 mL and 6 mL RAVICTI 3 times daily (13.2 g/day and 19.8 g/day, respectively) for 3 days, a dose-dependent increase in non-serious nervous system adverse reactions were observed. In subjects who had nervous system adverse reactions, plasma PAA concentrations, which were measured on Day 3 per protocol and not always at onset of symptoms, ranged from 8 to 56 micrograms/mL with 4 mL RAVICTI 3 times daily and from 31 to 242 micrograms/mL with 6 mL RAVICTI 3 times daily.
In clinical trials in patients with UCDs who had been on sodium phenylbutyrate prior to administration of RAVICTI, adverse reactions of headache, fatigue, symptoms of peripheral neuropathy, seizures, tremor and/or dizziness were reported. No correlation between plasma PAA concentration and neurologic symptoms was identified but plasma PAA concentrations were generally not consistently measured at the time of neurologic symptom occurrence [see Clinical Pharmacology (12.3)].
If symptoms of vomiting, nausea, headache, somnolence or confusion are present in the absence of high ammonia or other intercurrent illness which explains these symptoms, consider the potential for PAA neurotoxicity which may need reduction in the RAVICTI dosage [see Dosage and Administration (2.4)].
5.2 Pancreatic Insufficiency or Intestinal Malabsorption
Exocrine pancreatic enzymes hydrolyze RAVICTI in the small intestine, separating the active moiety, phenylbutyrate, from glycerol. This process allows phenylbutyrate to be absorbed into the circulation. Low or absent pancreatic enzymes or intestinal disease resulting in fat malabsorption may result in reduced or absent digestion of RAVICTI and/or absorption of phenylbutyrate and reduced control of plasma ammonia. Monitor ammonia levels closely in patients with pancreatic insufficiency or intestinal malabsorption.
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are described elsewhere in the labeling:
- Neurotoxicity [see Warnings and Precautions (5.1)]
- Pancreatic insufficiency or Intestinal Malabsorption [see Warnings and Precautions (5.2)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Assessment of adverse reactions was based on exposure of 45 adult patients (31 female and 14 male) with UCD subtype deficiencies of ornithine transcarbamylase (OTC, n=40), carbamoyl phosphate synthetase (CPS, n=2), and argininosuccinate synthetase (ASS, n=1) in a randomized, double-blind, active-controlled (RAVICTI vs sodium phenylbutyrate), crossover, 4-week study (Study 1) that enrolled patients 18 years of age and older [see Clinical Studies (14.1)]. One of the 45 patients received only sodium phenylbutyrate prior to withdrawing on day 1 of the study due to an adverse reaction.
The most common adverse reactions (occurring in at least 10% of patients) reported during short-term treatment with RAVICTI were diarrhea, flatulence, and headache. Table 1 summarizes adverse reactions occurring in 2 or more patients treated with RAVICTI or sodium phenylbutyrate (incidence of at least 4% in either treatment arm).
| Number (%) of Patients in Study 1 | ||
|---|---|---|
| Sodium Phenylbutyrate (N = 45) | RAVICTI (N = 44) |
|
| Diarrhea | 3 (7) | 7 (16) |
| Headache | 4 (9) | 6 (14) |
| Flatulence | 1 (2) | 6 (14) |
| Abdominal pain | 2 (4) | 3 (7) |
| Vomiting | 2 (4) | 3 (7) |
| Decreased appetite | 2 (4) | 3 (7) |
| Fatigue | 1 (2) | 3 (7) |
| Dyspepsia | 3 (7) | 2 (5) |
| Nausea | 3 (7) | 1 (2) |
| Dizziness | 4 (9) | 0 |
| Abdominal discomfort | 3 (7) | 0 |
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of RAVICTI. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure:
- Abnormal body odor, including from skin, hair and urine
- Retching and gagging
- Dysgeusia or burning sensation in mouth
7. Drug Interactions
8. Use In Specific Populations
8.5 Geriatric Use
Clinical studies of RAVICTI did not include sufficient numbers of subjects 65 years of age and older to determine whether they respond differently than younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment
The efficacy and safety of RAVICTI in patients with renal impairment are unknown. Monitor ammonia levels closely when starting patients with impaired renal function on RAVICTI.
8.7 Hepatic Impairment
No studies were conducted in patients with UCDs and hepatic impairment. Because conversion of PAA to PAGN occurs in the liver, patients with hepatic impairment may have reduced conversion capability and higher plasma PAA and PAA to PAGN ratio [see Clinical Pharmacology (12.3)]. Therefore, dosage for patients with moderate to severe hepatic impairment should be started at the lower end of the recommended dosing range and should be kept on the lowest dose necessary to control their ammonia levels [see Dosage and Administration (2.5)].
10. Overdosage
While there is no experience with overdosage in human clinical trials, PAA, a toxic metabolite of RAVICTI, can accumulate in patients who receive an overdose [see Warnings and Precautions (5.1)].
If over-exposure occurs, call your Poison Control Center at 1-800-222-1222 for current information on the management of poisoning or overdosage.
11. Ravicti Oral Liquid Description
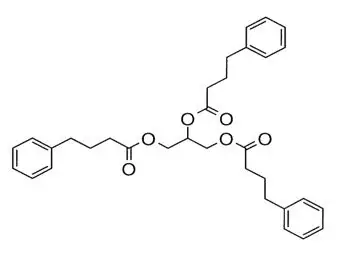
RAVICTI (glycerol phenylbutyrate) is a clear, colorless to pale yellow oral liquid. It is insoluble in water and most organic solvents, and it is soluble in dimethylsulfoxide (DMSO) and greater than 65% acetonitrile.
Glycerol phenylbutyrate is a nitrogen-binding agent. It is a triglyceride containing 3 molecules of PBA linked to a glycerol backbone, the chemical name of which is benzenebutanoic acid, 1′, 1′ ′ –(1,2,3-propanetriyl) ester with a molecular weight of 530.67. It has a molecular formula of C33H38O6. The structural formula is:
12. Ravicti Oral Liquid - Clinical Pharmacology
12.1 Mechanism of Action
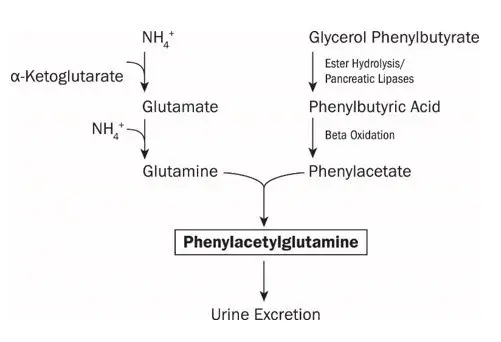
UCDs are inherited deficiencies of enzymes or transporters necessary for the synthesis of urea from ammonia (NH3, NH4+). Absence of these enzymes or transporters results in the accumulation of toxic levels of ammonia in the blood and brain of affected patients. RAVICTI is a triglyceride containing 3 molecules of PBA. PAA, the major metabolite of PBA, is the active moiety of RAVICTI. PAA conjugates with glutamine (which contains 2 molecules of nitrogen) via acetylation in the liver and kidneys to form PAGN, which is excreted by the kidneys (Figure 1). On a molar basis, PAGN, like urea, contains 2 moles of nitrogen and provides an alternate vehicle for waste nitrogen excretion.
Figure 1: RAVICTI Mechanism of Action
12.3 Pharmacokinetics
Absorption
RAVICTI is a pro-drug of PBA. Upon oral ingestion, PBA is released from the glycerol backbone in the gastrointestinal tract by lipases. PBA derived from RAVICTI is further converted by β-oxidation to PAA.
In healthy, fasting adult subjects receiving a single oral dose of 2.9 mL/m2 of RAVICTI, peak plasma levels of PBA, PAA, and PAGN occurred at 2 hours, 4 hours, and 4 hours, respectively. Upon single-dose administration of RAVICTI, plasma concentrations of PBA were quantifiable in 15 of 22 participants at the first sample time postdose (0.25 hours). Mean maximum concentration (Cmax) for PBA, PAA, and PAGN was 37.0 micrograms/mL, 14.9 micrograms/mL, and 30.2 micrograms/mL, respectively. In healthy subjects, intact glycerol phenylbutyrate was detected in plasma. While the study was inconclusive, the incomplete hydrolysis of glycerol phenylbutyrate cannot be ruled out.
In healthy subjects, the systemic exposure to PAA, PBA, and PAGN increased in a dose-dependent manner. Following 4 mL of RAVICTI 3 times a day for 3 days, the mean Cmax and AUC were 66 micrograms/mL and 930 micrograms∙h/mL for PBA and 28 micrograms/mL and 942 micrograms∙h/mL for PAA, respectively. In the same study, following 6 mL of RAVICTI three times a day for 3 days, mean Cmax and AUC were 100 micrograms/mL and 1400 micrograms∙h/mL for PBA and 65 μg/mL and 2064 micrograms∙h/mL for PAA, respectively.
In adult patients with UCDs receiving multiple doses of RAVICTI, maximum plasma concentrations at steady state (Cmax,ss) of PBA, PAA, and PAGN occurred at 8 hours, 12 hours, and 10 hours, respectively, after the first dose in the day. Intact glycerol phenylbutyrate was not detectable in plasma in patients with UCDs.
In clinical studies of RAVICTI in patients with UCDs, the peak observed PAA concentrations by age group are shown in Table 2.
| Age Range | RAVICTI Dose | Mean Peak PAA Concentration*
(SD) | Median Peak PAA Concentration *
(Range) |
|---|---|---|---|
|
|||
| Less than 2 months (n=16) | 3.1 to 12.7 mL/m2/day (3.4 to 14 g/m2/day) | 257 (162) | 205 (96 to 707) |
| 2 months to less than 2 years (n=17) | 3.3 to 12.3 mL/m2/day (3.7 to 13.5 g/m2/day) | 142 (299) | 35 (1 to 1215) |
| 2 years to 17 years (n=53) | 1.4 to 13.7 mL/m2/day (1.5 to 15.1 g/m2/day) | 70 (79) | 50 (1 to 410) |
| Adults (n=43) | 0.6 to 14 mL/m2/day (0.7 to 15.4 g/m2/day) | 39 (40) | 25 (1.6 to 178) |
Drug Interaction Studies
In vitro PBA or PAA did not induce CYP1A2, suggesting that in vivo drug interactions via induction of CYP1A2 is unlikely.
In in vitro studies, PBA at a concentration of 800 micrograms/mL caused greater than 60% reversible inhibition of cytochrome P450 isoenzymes CYP2C9, CYP2D6, and CYP3A4/5 (testosterone 6β-hydroxylase activity). The in vitro study suggested that in vivo drug interactions with substrates of CYP2D6 cannot be ruled out. The inhibition of CYP isoenzymes 1A2, 2C8, 2C19, and 2D6 by PAA at the concentration of 2.8 mg/mL was observed in vitro. Clinical implication of these results is unknown.
14. Clinical Studies
14.1 Clinical Studies in Adult Patients with UCDs
Active-Controlled, 4-Week, Noninferiority Study (Study 1)
A randomized, double-blind, active-controlled, crossover, noninferiority study (Study 1) compared RAVICTI to sodium phenylbutyrate by evaluating ammonia levels in patients with UCDs who had been on sodium phenylbutyrate prior to enrollment for control of their UCD. Patients were required to have a confirmed diagnosis of UCD involving deficiencies of CPS, OTC, or ASS, confirmed via enzymatic, biochemical, or genetic testing. Patients had to have no clinical evidence of hyperammonemia at enrollment and were not allowed to receive drugs known to increase ammonia levels (e.g., valproate), increase protein catabolism (e.g., corticosteroids), or significantly affect renal clearance (e.g., probenecid).
The primary endpoint was the 24-hour AUC (a measure of exposure to ammonia over 24 hours) for venous ammonia on days 14 and 28 when the drugs were expected to be at steady state. Statistical noninferiority would be established if the upper limit of the 2-sided 95% CI for the ratio of the geometric means (RAVICTI/sodium phenylbutyrate) for the endpoint was 1.25 or less.
Forty-five patients were randomized 1:1 to 1 of 2 treatment arms to receive either
- –
- Sodium phenylbutyrate for 2 weeks → RAVICTI for 2 weeks; or
- –
- RAVICTI for 2 weeks → sodium phenylbutyrate for 2 weeks.
Sodium phenylbutyrate or RAVICTI were administered three times daily with meals. The dose of RAVICTI was calculated to deliver the same amount of PBA as the sodium phenylbutyrate dose the patients were taking when they entered the study. Forty-four patients received at least 1 dose of RAVICTI in the study.
Patients adhered to a low-protein diet and received amino acid supplements throughout the study. After 2 weeks of dosing, by which time patients had reached steady state on each treatment, all patients had 24 hours of ammonia measurements.
Demographic characteristics of the 45 patients enrolled in Study 1 were as follows: mean age at enrollment was 33 years (range: 18 to 75 years); 69% were female; 33% had adult-onset disease; 89% had OTC deficiency; 7% had ASS deficiency; 4% had CPS deficiency.
RAVICTI was non-inferior to sodium phenylbutyrate with respect to the 24-hour AUC for ammonia. Forty-four patients were evaluated in this analysis. Mean 24-hour AUCs for ammonia during steady-state dosing were 866 micromol∙h/L and 977 micromol∙h/L with RAVICTI and sodium phenylbutyrate, respectively. The ratio of geometric means was 0.91 [95% CI 0.8, 1.04].
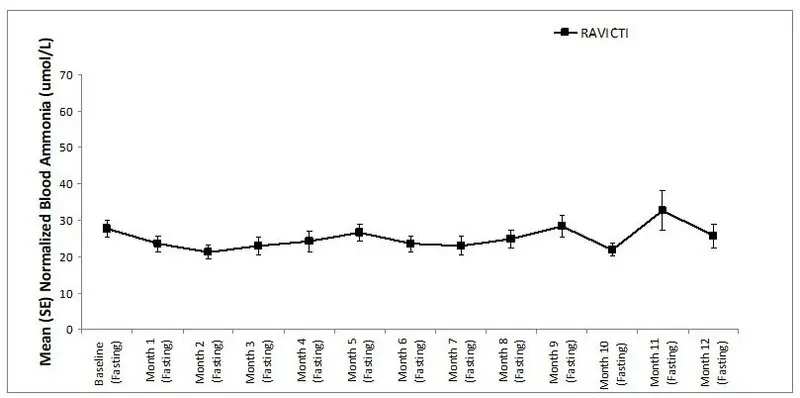
The mean ammonia levels over 24-hours after 2 weeks of dosing (on day 14 and 28) in the double-blind short-term study (Study 1) are displayed in Figure 2 below. The mean and median maximum ammonia levels (Cmax) over 24 hours and 24-hour AUC for ammonia are summarized in Table 3. Ammonia values across different laboratories were normalized to a common normal range of 9 to 35 micromol/L using the following formula after standardization of the units to micromol/L:
Normalized ammonia (micromol/L) = ammonia readout in micromol/L × (35/ULN of a laboratory reference range specified for each assay)
Figure 2: Ammonia Levels in Adult Patients with UCDs in Short-Term Treatment Study 1
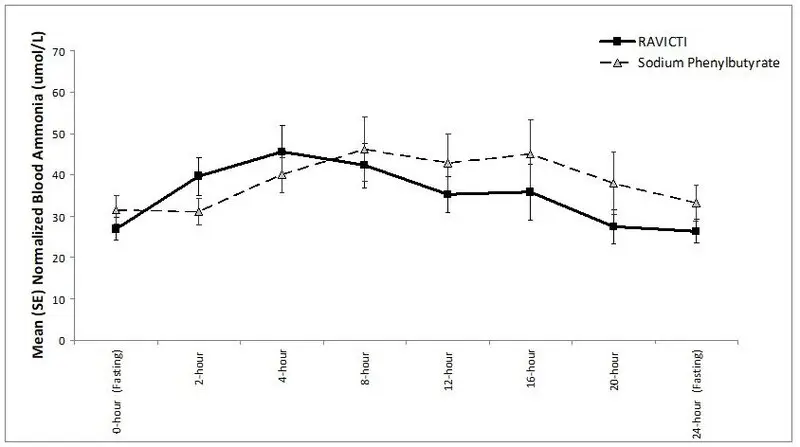
| Timepoint | Ammonia (n=44) | |
|---|---|---|
| Mean (SD) | Median (min, max) | |
| Daily Cmax (micromol/L) | ||
| RAVICTI | 61 (46) | 51 (12, 245) |
| Sodium phenylbutyrate | 71 (67) | 46 (14, 303) |
| 24-Hour AUC (micromol∙h/L) | ||
| RAVICTI | 866 (661) | 673 (206, 3351) |
| Sodium phenylbutyrate | 977 (865) | 653 (302, 4666) |
14.2 Clinical Studies in Pediatric Patients 2 Years to 17 Years of Age with UCDs
The efficacy of RAVICTI in pediatric patients 2 years to 17 years of age with UCDs was evaluated in 2 fixed-sequence, open-label, sodium phenylbutyrate to RAVICTI switchover studies (Studies 3 and 4). Study 3 was 7 days in duration and Study 4 was 10 days in duration.
These studies compared ammonia levels of patients on RAVICTI to ammonia levels of patients on sodium phenylbutyrate in 26 pediatric patients between 2 months and 17 years of age with UCDs. Four patients less than 2 years of age were excluded from this analysis due to insufficient data. The dose of RAVICTI was calculated to deliver the same amount of PBA as the dose of sodium phenylbutyrate that patients were taking when they entered the trial. Sodium phenylbutyrate or RAVICTI were administered in divided doses with meals. Patients adhered to a low-protein diet throughout the study. After a dosing period with each treatment, all patients underwent 24 hours of venous ammonia measurements, as well as blood and urine pharmacokinetic assessments.
UCD subtypes included OTC (n=12), ASL (n=8), and ASS deficiency (n=2), and patients received a mean RAVICTI dose of 8 mL/m2/day (8.8 g/m2/day), with doses ranging from 1.4 to 13.1 mL/m2/day (1.5 to 14.4 g/m2/day). Doses in these patients were based on previous dosing of sodium phenylbutyrate.
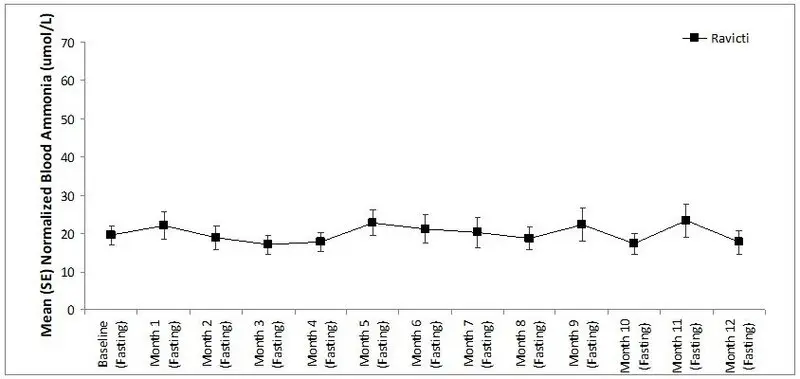
The 24-hour AUCs for ammonia (AUC0-24h) in 11 pediatric patients 6 years to 17 years of age with UCDs (Study 3) and 11 pediatric patients 2 years to 5 years of age with UCDs (Study 4) were similar between treatments. In pediatric patients 6 years to 17 years of age, the ammonia AUC0-24h was 604 micromol∙h/L vs 815 micromol∙h/L on RAVICTI vs sodium phenylbutyrate, respectively. In patients between 2 years and 5 years of age with UCDs, the ammonia AUC0-24h was 632 micromol∙h/L vs 720 micromol∙h/L on RAVICTI versus sodium phenylbutyrate, respectively.
The mean ammonia levels over 24 hours in open-label, short-term Studies 3 and 4 at common time points are displayed in Figure 4. Ammonia values across different laboratories were normalized to a common normal range of 9 to 35 micromol/L using the following formula after standardization of the units to micromol/L:
Normalized ammonia (micromol/L) = ammonia readout in micromol/L × (35/ULN of a laboratory reference range specified for each assay)
Figure 4: Ammonia Levels in Pediatric Patients 2 Years to 17 Years of Age with UCDs in Short-Term Treatment Studies 3 and 4
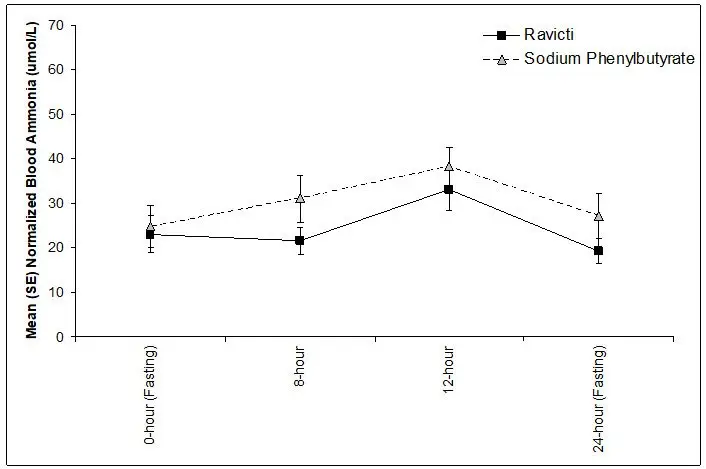
14.3 Clinical Studies in Pediatric Patients Less Than 2 Years of Age with UCDs
The efficacy of RAVICTI in pediatric patients less than 2 years of age with UCDs was evaluated in uncontrolled, open label studies (Studies 4/4E, 5 [see Clinical Studies (14.2)] and 6). A total of 17 pediatric patients with UCDs aged 2 months to less than 2 years participated in Studies 4/4E, 5 and 6. Study 6 enrolled 16 pediatric patients less than 2 months of age.
Uncontrolled, Open-Label Study in Pediatric Patients Less Than 2 Years of Age (Study 6)
Study 6 was an uncontrolled, open label study in pediatric patients less than 2 years of age. The primary efficacy endpoint was successful transition to RAVICTI within a period of 4 days followed by 3 days of observation for a total of 7 days, where successful transition was defined as no signs and symptoms of hyperammonemia and a venous ammonia level less than 100 micromol/L. Ammonia levels were monitored for up to 4 days during transition and on day 7.
Pediatric Patients Less than 2 Months of Age
A total of 16 pediatric patients less than 2 months of age participated in Study 6. Median age at enrollment was 0.5 months (range: 0.1 to 2 months). Eight patients had OTC deficiency, 7 patients had ASS deficiency, and 1 patient had ASL deficiency. Ten of the 16 patients transitioned from sodium phenylbutyrate to RAVICTI within 3 days of treatment and their initial dosage of RAVICTI was calculated to deliver the same amount of phenylbutyrate as the sodium phenylbutyrate dosage administered prior to RAVICTI dosing. Three of the 16 patients were treatment-naïve and started RAVICTI at dosages of 9, 9.4, and 9.6 mL/m2/day. The remaining 3 of the 16 patients transitioned from intravenous sodium benzoate and sodium phenylacetate to RAVICTI within 3 days of treatment and their initial dosages of RAVICTI were 10.4, 10.9, and 10.9 mL/m2/day.
Of the 16 patients, 16, 14, 12, 6, and 3 patients were treated for 1, 3, 6, 12, and 18 months, respectively.
After the initial 7-day transition period, patients received a mean RAVICTI dosage of 8 mL/m2/day (8.8 g/m2/day), with doses ranging from 3.1 to 12.7 mL/m2/day (3.4 to 14 g/m2/day). The frequency of dosing varied throughout the study. The majority of patients were dosed three times per day with feeding. No patients discontinued during the 7-day transition phase. Ammonia values across different laboratories were normalized (transformed) to a common normal pediatric range of 28 to 57 micromol/L for comparability.
During the safety extension phase (months 1-24), venous ammonia levels were monitored monthly for the first 6 months of treatment and every 3 months thereafter until the patients terminated or completed the study. During the safety extension phase, 1 patient discontinued from the study due to an adverse event (increased hepatic enzymes), 2 patients were withdrawn from the study by their parent/guardian, and 4 patients discontinued from the study early to undergo a liver transplant (protocol-defined discontinuation criterion). The normalized ammonia levels in pediatric patients with available values (which varied by month of treatment) in Study 6 in patients less than 2 months of age are shown in Table 4.
| Month | N (patients with available ammonia level) | Normalized Ammonia (micromol/L)† | |
|---|---|---|---|
| Mean (SD) | Median (Min, Max) |
||
|
|||
| 1 | 15 | 71 (52) | 60 (18, 227) |
| 2 | 11 | 58 (40) | 50 (16, 168) |
| 3 | 14 | 53 (34) | 46 (11, 122) |
| 4 | 11 | 94 (106) | 64 (35, 407) |
| 5 | 10 | 52 (18) | 57 (27, 86) |
| 6 | 9 | 49 (24) | 42 (22, 91) |
| 9 | 8 | 56 (34) | 45 (22, 122) |
| 12 | 6 | 35 (17) | 36 (11, 60) |
| 15 | 4 | 52 (12) | 52 (39, 67) |
| 18 | 3 | 64 (14) | 63 (50, 78) |
| 24 | 9 | 63 (29) | 72 (23, 106) |
Five patients (all less than 1 month of age) experienced a total of 7 hyperammonemic crises defined as in Study 4/4E and 5. Hyperammonemic crises were precipitated by upper respiratory tract infection (2 events), change in diet (1 event), or had no identified precipitating event (4 events).
16. How is Ravicti Oral Liquid supplied
RAVICTI® (glycerol phenylbutyrate) oral liquid 1.1 g/mL is supplied in multi-use, 25-mL glass bottles. The bottles are supplied in the following configurations:
- NDC 75987-050-06: Single 25-mL bottle per carton
- NDC 75987-050-07: Four 25-mL bottles per carton
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| RAVICTI
glycerol phenylbutyrate liquid |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Horizon Therapeutics, Inc. (033470838) |