Drug Detail:Rebinyn (Coagulation factor ix [ koe-ag-yoo-lay-shun-fak-tor-nine ])
Drug Class: Miscellaneous coagulation modifiers
Highlights of Prescribing Information
REBINYN® (Coagulation Factor IX (Recombinant), GlycoPEGylated)
lyophilized powder for solution for intravenous injection
Initial U.S. Approval: 2017
Recent Major Changes
Indications and Usage (1) ………………………………………07/2022
Dosage and Administration (2.1)…………………………………07/2022
Indications and Usage for Rebinyn
REBINYN, Coagulation Factor IX (Recombinant), GlycoPEGylated, is a recombinant DNA-derived coagulation Factor IX concentrate indicated for use in adults and children with hemophilia B (congenital Factor IX deficiency) for:
- •
- On-demand treatment and control of bleeding episodes
- •
- Perioperative management of bleeding
- •
- Routine prophylaxis to reduce the frequency of bleeding episodes
Limitations of Use: REBINYN is not indicated for immune tolerance induction in patients with hemophilia B (1).
Rebinyn Dosage and Administration
For intravenous infusion after reconstitution only (2).
- •
- Each carton and vial label for REBINYN states the actual Factor IX potency in international units (IU) (2.1).
- On-demand treatment and control of bleeding episodes:
- •
- 40 IU/kg body weight for minor and moderate bleeds, and 80 IU/kg body weight for major bleeds. Additional doses of 40 IU/kg can be given (2.1).
- Perioperative management:
- •
- Pre-operative dose of 40 IU/kg body weight for minor surgery, and 80 IU/kg body weight for major surgery. As clinically needed for the perioperative management of bleeding, repeated doses of 40 IU/kg (in 1-3 day intervals) within the first week after major surgery may be administered.
- •
- Frequency may be extended to once weekly after the first week until bleeding stops and healing is achieved (2.1).
- Routine prophylaxis:
- •
- 40 IU/kg body weight once weekly (2.1).
Dosage Forms and Strengths
REBINYN is available as a lyophilized powder in single-dose vials of 500, 1000, 2000, and 3000 IU (3).
Contraindications
Do not use in patients who have known hypersensitivity to REBINYN or its components, including hamster proteins (4).
Warnings and Precautions
- •
- Hypersensitivity reactions, including anaphylaxis, have occurred. Should hypersensitivity reactions occur, discontinue REBINYN and administer appropriate treatment (5.1).
- •
- Neutralizing antibodies (inhibitors) to Factor IX have occurred following administration of REBINYN. Perform an assay that measures Factor IX inhibitor concentration if bleeding is not controlled with the recommended dose of REBINYN or if plasma Factor IX activity level fails to increase as expected (5.2, 5.5).
- •
- The use of Factor IX- products has been associated with the development of thromboembolic complications (5.3).
- •
- Nephrotic syndrome has been reported following immune tolerance induction with Factor IX-containing products in hemophilia B patients with Factor IX inhibitors and a history of allergic reactions to Factor IX. (5.4)
- •
- Factor IX activity assay results may vary with the type of activated partial thromboplastin time reagent used (5.5).
Adverse Reactions/Side Effects
The most frequently reported adverse reactions (≥ 1%) in previously treated patients (PTPs) and previously untreated patients (PUPs) were itching and injection site reactions (6).
Additional frequently reported adverse reactions (≥ 1%) in PUPs included rash, Factor IX inhibition, hypersensitivity, and anaphylactic reaction (6).
In animals administered repeat doses of REBINYN, accumulation of polyethylene-glycol (PEG) was observed in the choroid plexus, pituitary, circumventricular organs, and cranial motor neurons (8.4 and 13.2). The potential clinical implications of these animal findings are unknown (6.3).
To report SUSPECTED ADVERSE REACTIONS, contact Novo Nordisk Inc. at 1-877-668-6777 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Use In Specific Populations
Pediatric Use: No dose adjustment is needed (8.4).
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 8/2022
Related/similar drugs
Hemgenix, Alprolix, NovoSeven RT, BeneFix, factor ix complex, IdelvionFull Prescribing Information
1. Indications and Usage for Rebinyn
REBINYN, Coagulation Factor IX (Recombinant), GlycoPEGylated, is a recombinant DNA-derived coagulation Factor IX concentrate indicated for use in adults and children with hemophilia B (congenital Factor IX deficiency) for:
- •
- On-demand treatment and control of bleeding episodes
- •
- Perioperative management of bleeding
- •
- Routine prophylaxis to reduce the frequency of bleeding episodes
Limitations of Use: REBINYN is not indicated for immune tolerance induction in patients with hemophilia B.
2. Rebinyn Dosage and Administration
For intravenous infusion after reconstitution only.
2.1 Dosing Guidelines
- •
- Dose and duration of treatment depend on the location and extent of bleeding, and the patient’s clinical condition.
- •
- If monitoring of Factor IX activity is performed, use a chromogenic assay or selected one-stage clotting assay validated for use with REBINYN [see Warnings and Precautions (5.5)].
- •
- Each carton and vial label for REBINYN states the actual Factor IX potency in IU.
On-demand Treatment and Control of Bleeding Episodes
REBINYN dosing for on-demand treatment and control of bleeding episodes is provided in Table 1.
Table 1: Dosing for On-demand Treatment and Control of Bleeding Episodes
|
Type of bleeding |
Recommended dose IU/kg body weight |
Additional information |
|
Minor and moderate For example: Uncomplicated joint bleeds, minor muscular bleeds, mucosal or subcutaneous bleeds |
40 |
A single dose should be sufficient for minor and moderate bleeds. Additional doses of 40 IU/kg can be given. |
|
Major For example: Intracranial, retroperitoneal, iliopsoas and neck bleeds, muscle bleeds with compartment syndrome and bleeds associated with a significant decrease in the hemoglobin level |
80 |
Additional doses of 40 IU/kg can be given. |
Perioperative Management
REBINYN dosing for perioperative management is provided in Table 2.
Table 2: Dosing for Perioperative Management
|
Type of surgical procedure |
Recommended dose IU/kg body weight |
Additional Information |
|
Minor For example: Implanting pumps in subcutaneous tissue, skin biopsies or simple dental procedures |
40 |
A single pre-operative dose should be sufficient. Additional doses can be given if needed. |
|
Major For example: Body cavity is entered, mesenchymal barrier is crossed, fascial plane is opened, organ is removed, normal anatomy is operatively altered |
80 |
Pre-operative dose |
|
40 |
As clinically needed for the perioperative management of bleeding, repeated doses of 40 IU/kg (in 1-3 day intervals) within the first week after major surgery may be administered.* Due to the long half-life of REBINYN, the frequency of dosing in the post-surgical setting may be extended to once weekly after the first week until bleeding stops and healing is achieved. |
*See 12.3 Pharmacokinetics, Table 8
Routine Prophylaxis
For prophylaxis use, the recommended dose is 40 IU/kg body weight once weekly.
Adjust dosing regimen based on individual patient’s bleeding pattern, and physical activity.
2.2 Reconstitution
- •
- Always wash hands and ensure that the area is clean before performing the reconstitution procedures.
- •
- Use aseptic technique during the reconstitution procedures.
- •
- If the patient uses more than one vial of REBINYN per infusion, reconstitute each vial according to the following instructions.
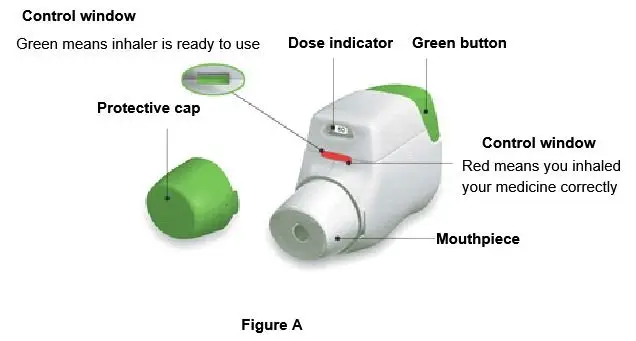
Overview of REBINYN Package
The instructions below serve as a general guideline for reconstitution of REBINYN. For full instructions, refer to the FDA-approved patient information and Instructions for Use.
Reconstitution
- 1.
- Bring the REBINYN vial and the pre-filled diluent syringe to room temperature.
- 2.
- Remove the plastic cap from the REBINYN vial.
- 3.
- Wipe the rubber stopper on the vial with a sterile alcohol swab and allow it to dry prior to use.
- 4.
- Remove the protective paper from the vial adapter. Do not remove the vial adapter from the protective cap.
- 5.
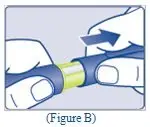
- Place the vial on a flat and solid surface. While holding the protective cap, place the vial adapter over the REBINYN vial and press down firmly on the protective cap until the vial adapter spike penetrates the rubber stopper.
- 6.
- Remove the protective cap from the vial adapter.
- 7.
- Grasp the plunger rod as shown in the diagram. Attach the plunger rod to the syringe by holding the plunger rod by the wide top end. Turn the plunger rod clockwise into the rubber plunger inside the pre-filled diluent syringe until resistance is felt.
- 8.
- Break off the syringe cap from the pre-filled diluent syringe by snapping the perforation of the cap.
- 9.
- Connect the pre-filled diluent syringe to the vial adapter by turning it clockwise until it is secured.
- 10.
- Push the plunger rod to slowly inject all the diluent into the vial.
- 11.
- Without removing the syringe, gently swirl the REBINYN vial until all of the powder is dissolved.
- 12.
- Administer the REBINYN solution immediately [see Administration (2.3)]. If not used immediately after reconstitution, store the solution in the vial with the vial adapter and the syringe attached, at room temperature ≤ 86°F (30°C). Do not store for longer than 4 hours.
2.3 Administration
For intravenous infusion only.
- •
- Accidental needle stick with a needle contaminated with blood can transmit infectious viruses including HIV (AIDS) and hepatitis. If a needle stick occurs, obtain immediate medical attention. Place needles in a sharps container after single use.
- •
- Inspect the reconstituted REBINYN solution visually prior to administration [see Description (11)]. The solution should be clear and have no particles. Do not use if particulate matter or discoloration is observed.
- •
- Do not administer REBINYN in the same tubing or container with other medicinal products.
- 1.
- Invert the REBINYN vial and slowly draw the solution into the syringe.
- 2.
- Detach the syringe from the vial adapter by turning the syringe counterclockwise.
- 3.
- Attach the syringe to the luer end of an infusion needle set.
- 4.
- Infuse the reconstituted REBINYN intravenously slowly over 1 to 4 minutes.
- 5.
- After infusion, safely dispose of the syringe with the infusion set, the vial with the vial adapter, any unused REBINYN, and other waste materials.
Caution: The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector.
Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave® /MicroClave®, InVision-Plus®, InVision-Plus CS®, Invision-Plus Junior®, Bionector®), and their use can damage the connector and affect administration. To administer REBINYN through incompatible needleless connectors, withdraw the reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
If you encounter any problems with attaching the pre-filled histidine-diluent syringe to any Luer‐lock compatible device, please contact Novo Nordisk at (844) 303-4448.
3. Dosage Forms and Strengths
REBINYN is available as a white to off-white lyophilized powder in single-dose vials containing nominally 500, 1000, 2000, or 3000 IU per vial. Each carton and vial label for REBINYN states the actual Factor IX potency in IU.
After reconstitution with 4 mL of histidine diluent, the reconstituted solution contains approximately 125, 250, 500, or 750 IU per mL of REBINYN respectively.
4. Contraindications
REBINYN is contraindicated in patients who have known hypersensitivity to REBINYN or its components (including hamster proteins) [see Warnings and Precautions (5.1) and Description (11)]
5. Warnings and Precautions
5.1 Hypersensitivity Reactions
Allergic-type hypersensitivity reactions, including anaphylaxis, have occurred with REBINYN. The product may contain traces of hamster proteins which in some patients may cause allergic reactions. Signs of allergic reactions, which can progress to anaphylaxis, may include angioedema, chest tightness, difficulty breathing, wheezing, urticaria, and itching. Observe patients for signs and symptoms of acute hypersensitivity reactions, particularly during the early phases of exposure to the product. Discontinue use of REBINYN if allergic- or anaphylactic - type reactions occur, and initiate appropriate treatment.
5.2 Inhibitors
The formation of inhibitors (neutralizing antibodies) to Factor IX has occurred following REBINYN. If expected plasma factor IX activity levels are not attained, or if bleeding is not controlled as expected with the administered dose, perform an assay that measures Factor IX inhibitor concentration. Monitor all patients using clinical observations and laboratory tests for the development of inhibitors [see Warnings and Precautions (5.5)].
An association between the development of Factor IX inhibitors and allergic reactions has been reported. Evaluate patients experiencing allergic reactions for the presence of an inhibitor. Patients with Factor IX inhibitors may be at an increased risk of severe allergic reactions with subsequent exposure to Factor IX.
5.3 Thrombotic Events
The use of Factor IX-containing products has been associated with thromboembolic complications. Due to the potential risk of thromboembolic complications, monitor patients for early signs of thrombotic and consumptive coagulopathy when administering this product to patients with liver disease, post-operatively, to newborn infants, or to patients at risk of thrombosis or disseminated intravascular coagulation (DIC). In each of these situations, the benefit of treatment with REBINYN should be weighed against the risk of these complications.
5.4 Nephrotic Syndrome
Nephrotic syndrome has been reported following immune tolerance induction therapy with Factor IX products in hemophilia B patients with Factor IX inhibitors, often with a history of allergic reactions to Factor IX. The safety and efficacy of using REBINYN for immune tolerance induction have not been established.
5.5 Monitoring Laboratory Tests
If monitoring of Factor IX activity is performed, use a chromogenic assay or selected one-stage clotting assay validated for use with REBINYN [see Dosage and Administration (2)].
The one-stage clotting assay results can be significantly affected by the type of activated partial thromboplastin time (aPTT) reagent used, which can result in over- or under-estimation of Factor IX activity. Avoid the use of silica-based reagents, as some may overestimate the activity of REBINYN. If a validated one-stage clotting or chromogenic assay is not available locally, then use of a reference laboratory is recommended.
If bleeding is not controlled with the recommended dose of REBINYN, or if the expected Factor IX activity levels in plasma are not attained, then perform a Bethesda assay to determine if Factor IX inhibitors are present.
6. Adverse Reactions/Side Effects
Common adverse reactions (incidence ≥ 1%) in PTPs reported in clinical trials for REBINYN were itching and injection site reactions. Common adverse reactions (incidence ≥ 1%) in PUPs reported in clinical trials for REBINYN were rash, FIX inhibitors, hypersensitivity, itching, injection site reaction, and anaphylactic reaction.
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in clinical practice.
Previously Treated Patients (PTPs)
In five multicenter, prospective, non-controlled, open-label clinical trials, 115 PTPs [0 to 6 years old: 12 subjects (10%); 7 to 12 years old: 13 subjects (11%); 13 to 17 years old: 18 subjects (16%); ≥18 years old: 72 subjects (63%)] received at least one dose of REBINYN as part of routine prophylaxis, on-demand treatment of bleeding episodes, perioperative management of major and minor surgery, or pharmacokinetic evaluation [see Clinical Studies (14)]. A PTP was defined as a subject with a history of at least 150 exposure days to other Factor IX products (adolescent/adult subjects) or 50 exposure days to other Factor IX products (pediatric subjects), and no history of inhibitors. A total of 15,167 injections were administered over a median of 733 days (range: 29- 2951 days), equivalent to 15,137 exposure days and 292 patient-years.
Adverse reactions in PTPs are listed in Table 3.
- Table 3: Summary of Adverse Reactions in Previously Treated Patients
|
System Organ Class |
Adverse Reaction |
Number of subjects (%) N=115 |
|
General disorders and administration site conditions |
Injection site reactions |
4 (4) |
|
Immune system disorders |
Hypersensitivity |
1 (1) |
|
Skin and subcutaneous tissue disorders |
Itching |
3 (3) |
Previously Untreated Patients (PUPs)
In one multicenter, prospective, non-controlled, open-label clinical trial conducted in PUPs, 50 subjects (≤6 years of age) received at least one dose of REBINYN [see Clinical Studies (14)]. A PUP was defined as a subject previously untreated or exposed to FIX-containing products less than or equal to 3 exposure days (5 previous exposures to blood components was acceptable). A total of 6,737 injections were administered over a median of 996 days (range: 61- 2,233 days), equivalent to 6,709 exposure days and 142 patient-years.
Adverse reactions in PUPs are listed in Table 4.
Table 4: Summary of Adverse Reactions in Previously Untreated Patients
|
System Organ Class |
Adverse Reaction |
Number of subjects (%) N=50 |
|
Blood and lymphatic system disorders |
Factor IX inhibition |
4 (8) |
|
General disorders and administration site conditions |
Injection site reaction |
1 (2) |
|
Immune system disorders |
Anaphylactic reaction Hypersensitivty |
1 (2) 3 (6) |
|
Skin and subcutaneous tissue disorders |
Rash Itching |
9 (18) 2 (4) |
6.2 Immunogenicity
Subjects were monitored for inhibitory antibodies to factor IX prior to dosing, on a monthly basis for the first three months, every two months up to one year, every three months for an additional year, and then every 6 months until end of trial.
No inhibitors were reported in the clinical trials in previously treated patients.
In an ongoing trial in previously untreated patients, one anaphylactic reaction has occurred with development of a factor IX inhibitor following treatment with REBINYN. Inhibitor development and anaphylactic reactions are more likely to occur during the early phases of factor IX replacement therapy [see Warnings and Precautions (5.1, 5.2)].
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
6.3 Neurologic Considerations
Animals administered repeat doses of REBINYN showed accumulation of PEG in the choroid plexus, pituitary, circumventricular organs, and cranial motor neurons [see Use in Specific Populations (8.4) and Animal Toxicology and/or Pharmacology (13.2)]. The potential clinical implications of these animal findings are unknown.
In the pediatric studies, 47 PUPs and 25 PTPs receiving routine prophylaxis with REBINYN at a weekly dose of 40 IU/kg were followed for central nervous system (CNS)-related ADRs for 6 and 8 years, respectively. The median duration of follow up of ADRs in the PUP and PTP studies were 2 and 7 years, respectively. Furthermore, neurological examinations were prospectively conducted in 44 PUPs and 17 PTPs with a median follow up of 2 years, and neurocognitive assessments were prospectively performed in 38 PUPs and 16 PTPs with a median follow up of 1 year.
Although no clear clinical implications of the animal findings are known and no clear clinical neurologic or neurocognitive safety signal has emerged, the physician should consider whether the patient is vulnerable to cognitive impairment, such as infants and children who have developing brains, and patients who are cognitively impaired. Factors such as duration of use, cumulative dose, age of the patient and co-morbidities that may increase risk of adverse neurologic and/or neurocognitive events should be considered when prescribing REBINYN. Report adverse neurocognitive and neurologic reactions.
6.4 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of REBINYN. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Blood and lymphatic system disorders: Factor IX inhibitor development.
8. Use In Specific Populations
8.1 Pregnancy
Risk Summary
There are no data with REBINYN use in pregnant women to determine whether there is a drug-associated risk. Animal reproduction studies have not been conducted with REBINYN. It is unknown whether REBINYN can cause fetal harm when administered to a pregnant woman or can affect fertility. In the U.S. general population, the estimated background risk of major birth defect and miscarriage in clinically recognized pregnancies is 2-4% and 15-20%, respectively.
8.2 Lactation
Risk Summary
There is no information regarding the presence of REBINYN in human milk, the effect on the breastfed infant, and the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for REBINYN and any potential adverse effects on the breastfed infant from REBINYN or from the underlying maternal condition.
8.4 Pediatric Use
Safety and efficacy of REBINYN were evaluated in four clinical trials that included 43 PTPs and in one clinical trial that included 50 pediatric PUPs [see Adverse Reactions (6) and Clinical Studies (14)]. Twelve of these subjects were ≤ 6 years of age; 13 subjects were 7 to 12 years of age; and 18 subjects were 13 to 17 years of age. Pharmacokinetic parameters were evaluated for 28 of the pediatric PTPs who were treated with REBINYN 40 IU/kg [see Clinical Pharmacology (12.3)].
Body weight-adjusted clearance was observed to be higher for pediatric subjects than for adult subjects. However, in clinical trials, no dose adjustment was needed in pediatric subjects who received a fixed dose of 40 IU/kg every week for routine prophylaxis.
Juvenile Animal Toxicity Data
A juvenile animal neurotoxicity study was conducted to evaluate the potential neurotoxicity of REBINYN when intravenously administered 120-1200 IU/kg/twice weekly in immature male rats from 3 to 13 weeks of age, followed by a 13-week treatment-free period. Accumulation of PEG was observed in the choroid plexus, pituitary, circumventricular organs, and the cranial motor neurons. PEG levels in these tissues increased with dose and dose duration (10 weeks) and remained detectable after the 13-week treatment-free period. Treatment-related PEG-positive vacuolated macrophages were observed in the pituitary. The accumulation of PEG was not associated with neurobehavioral changes, fertility, or functional effects.
8.5 Geriatric Use
Clinical studies of REBINYN did not include sufficient numbers of subjects age 65 and over to determine whether or not they respond differently than younger subjects.
Animals administered repeat doses of REBINYN showed accumulation of PEG in the choroid plexus, pituitary, circumventricular organs, and cranial motor neurons. [see Use in Specific Populations (8.4) and Animal Toxicology and/or Pharmacology (13.2)]. The potential clinical implications of these animal findings are unknown. No adverse neurologic effects of PEG have been reported in adults exposed to REBINYN during clinical trials, however, use in older adults with baseline cognitive dysfunction has not been fully evaluated [see Neurologic Considerations (6.3)].
11. Rebinyn Description
REBINYN is a sterile, non-pyrogenic, white to off-white lyophilized powder for reconstitution with the provided histidine diluent for intravenous infusion. After reconstitution, the solution appears as a clear and colorless to slightly yellow liquid, free from visible particles and contains the following excipients per mL: sodium chloride, 2.34 mg; histidine, 3.10 mg; sucrose, 10 mg; mannitol, 25 mg; polysorbate 80, 0.05 mg. REBINYN is available in single-dose vials containing the labeled amount of Factor IX activity, expressed in IU. Each vial contains nominally 500 IU, 1000 IU, 2000, or 3000 IU. REBINYN potency is assigned using an in vitro, activated partial thromboplastin time (aPTT)-based, one-stage clotting assay calibrated against the World Health Organization (WHO) international standard for Factor IX concentrates. REBINYN contains no preservatives.
REBINYN is a purified recombinant human Factor IX (rFIX) with a 40 kilodalton (kDa) polyethylene-glycol (PEG) conjugated to the protein. The 40 kDa PEG group is selectively attached to specific -N-linked glycans in the rFIX activation peptide, with mono-PEGylated rFIX as the predominant form of REBINYN. The rFIX protein in REBINYN consists of a gamma-carboxylated (Gla) domain, two EGF-like (epidermal growth factor) domains, an activation peptide (which is cleaved off upon activation), and a protease domain. Once activated, the resulting rFIX has structural and functional properties similar to those of endogenous activated Factor IX. The primary amino acid sequence in REBINYN is identical to the Thr148 allelic form of human plasma-derived Factor IX and consists of 415 amino acids. The average molecular weight of REBINYN is approximately 98 kDa and the molecular weight of the protein moiety alone is 56 kDa. The nominal specific activity of REBINYN is 144 IU/mg protein.
REBINYN is produced by recombinant DNA technology in Chinese Hamster Ovary (CHO) cells. No additives of human or animal origin are used in the cell culture, purification, conjugation, or formulation of REBINYN. The rFIX protein is purified by a series of chromatographic steps, including an affinity chromatography step using a monoclonal antibody (produced in CHO cells), to selectively isolate rFIX from the cell culture medium. The production process includes two dedicated viral clearance steps, namely a detergent treatment step for inactivation and a 20 nm filtration step for removal of viruses. The conjugation of the PEG-group is done by an enzymatic reaction during the purification process, followed by final purification of REBINYN.
12. Rebinyn - Clinical Pharmacology
12.1 Mechanism of Action
Patients with hemophilia B are deficient in coagulation Factor IX, which is required for effective hemostasis. Treatment with REBINYN temporarily replaces the missing coagulation Factor IX.
The Factor IX in REBINYN is conjugated to a 40-kDa polyethylene glycol molecule, which slows down its removal from the blood circulation.
12.2 Pharmacodynamics
The administration of REBINYN increases plasma levels of Factor IX and can temporarily correct the coagulation defect in hemophilia B patients, as reflected by a decrease in aPTT.
12.3 Pharmacokinetics
Pharmacokinetic (PK) parameters of REBINYN were evaluated in previously treated subjects, including a subset of subjects in the adult/adolescent trial and all subjects in the main phase of the pediatric trial [see Clinical Studies (14)]. PK samples were collected prior to dosing and at multiple time points up to 168 hours after dosing. The analysis of plasma samples was conducted using the one-stage clotting assay.
Steady state pharmacokinetic parameters for adolescents and adults following once-weekly prophylactic treatment of REBINYN 40 IU/kg are shown in Table 5.
Table 5: Steady-state pharmacokinetic parameters of REBINYN (40 IU/kg) in adolescents and adults (geometric mean (CV))
|
PK Parameter |
13-17 years N=3 |
≥ 18 years N=6 |
|
Half-life (hours) |
103.1 (14.2) |
114.9 (9.7) |
|
Incremental Recovery30min (IU/dL per IU/kg) |
1.82 (28.2) |
1.92 (19.6) |
|
AUC0-168 (IU*hours/dL) |
9072 (22) |
9280 (15) |
|
Clearance (mL/hour/kg) |
0.4 (16.7) |
0.4 (11.4) |
|
Mean residence time (hours) |
144.4 (15.3) |
158.1 (9.6) |
|
Vss (mL/kg) |
60.5 (31.1) |
65.8 (11.9) |
|
Factor IX activity 168 h after dosing (%) |
28.9 (18.6) |
32.4 (17.1) |
Abbreviations: AUC = area under plasma concentration-time curve; Vss= volume of distribution at steady state; CV=coefficient of variation.
The mean steady state pre-dose trough levels and post-dose peak levels across the clinical trials for all previously treated subjects are shown in Table 6.
Table 6: Factor IX peak and trough levels of REBINYN (40 IU/kg) by age at steady state
|
≤ 6 years N=12 |
7-12 years N=13 |
13-17 years N=9 |
≥18years N=20 |
|
|
Mean Factor IX peak level (%) (95% CI) |
65.5 |
71.4 (66.3; 77.0) |
82.8 |
97.9 (87.7; 109.3) |
|
Mean Factor IX trough level* (%) (95% CI) Min, Max** |
15.4 (13.2; 17.9) 9.2; 24.5 |
18.7 (16.2; 21.6) 8.3; 28.3 |
23.7 (19.9; 28.2) 18.6; 34.6 |
29.3 (26.0; 33.0) 21.3; 42.2 |
* Factor IX activity from samples collected at clinical site visits just prior to administration of next weekly dose
**Individual geometric mean trough values
Single-dose pharmacokinetic parameters of REBINYN in children, adolescents and adults are listed in Table 7.
Table 7: Single Dose Pharmacokinetic Parameters of REBINYN (40 IU/kg) in children, adolescents and adults (geometric mean (CV))
|
PK Parameter |
≤ 6 years N=12 |
7-12 years N=13 |
13-17 years N=3 |
≥18 years N=6 |
|
Half-life (hours) |
69.6 (15.8) |
76.3 (25.5) |
89.4 (24.1) |
83.0 (22.5) |
|
Incremental Recovery30min (IU/dL per IU/kg) |
1.51 (7.31) |
1.59 (16.2) |
1.96 (14.7) |
2.34 (11.3) |
|
AUCinf (IU*h/dL) |
4617 (14) |
5618 (19) |
7986 (35) |
9063 (16) |
|
Clearance (mL/hour/kg) |
0.8 (13.0) |
0.6 (21.9) |
0.5 (30.4) |
0.4 (14.7) |
|
Mean residence time (hours) |
95.4 (15.3) |
105.1 (24.2) |
124.2 (24.4) |
115.5 (21.8) |
|
Vss (mL/kg) |
72.3 (14.8) |
68.3 (21.7) |
58.6 (7.8) |
47.0 (15.9) |
|
Factor IX activity 168 h after dosing (%) |
8.4 (16.3) |
10.9 (18.9) |
14.6 (59.6) |
16.8 (30.6) |
Abbreviations: AUC = area under plasma concentration-time curve; Vss = volume of distribution at steady state; CV = coefficient of variation.
Pharmacokinetics were investigated in 9 subjects in the adult/adolescent trial, of which 5 were normal weight (body mass index (BMI) 18.5 to 24.9 kg/m2) and 4 were overweight (BMI 25 to <29.9 kg/m2). The pharmacokinetic parameters were not affected by BMI.
The Factor IX activity following 80 IU/kg infusion in major surgery is shown in Table 8.
Table 8: Factor IX activity following 80 IU/kg bolus for major surgery
|
30 minutes |
8 hours1 |
24 hours1 |
48 hours2 |
|
|
N=13 |
N=12 |
N=12 |
N=7 |
|
|
Factor IX activity (%) Median (Range) |
143 (123-224) |
138 (101-175) |
112 (62-146) |
73 (40-110) |
1 Excludes one subject with no Factor IX activity measurement obtained.
2 Excludes two subjects with no Factor IX activity measurement obtained and additionally 4 subjects re-dosed prior to second day after surgery for whom the Factor IX activity at 24 hours were 84%, 112%, 131% and 134%. The 48 hours measurement reflects a measurement on the 2nd day after surgery (range 47-57 hours).
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies in animals to evaluate the carcinogenic or genotoxic potential of REBINYN, or dedicated studies to determine the effects of REBINYN on fertility, have not been performed. In a juvenile rat study (3 to 13 week old male rats), fertility was unaffected following 9 weeks of twice weekly administrations of up to 1200 IU/kg [see Use in Specific Populations (8.4)].
13.2 Animal Toxicology and/or Pharmacology
REBINYN was intravenously administered in repeat-dose toxicity studies in adult immune-deficient rats (40-1200 IU/kg/week for 26 weeks), immune-competent monkeys (350-3750 IU/kg/week for 4 weeks), and juvenile immune-competent rats (120‑1200 IU/kg/twice weekly for 10 weeks). Accumulation of PEG was detected in epithelial cells of the choroid plexus in the brain of the adult rats and monkeys. This finding was not associated with morphological changes or abnormal clinical signs. In juvenile rats, more comprehensive assessment of brain tissues demonstrated PEG accumulation in the choroid plexus, pituitary, circumventricular organs, and the cranial motor neurons [see Use in Specific Populations (8.4)].
14. Clinical Studies
Safety and efficacy of REBINYN was evaluated in five multicenter, non-controlled, open-label trials in on-demand treatment of bleeding episodes, perioperative management of major and minor surgery, and routine prophylaxis or pharmacokinetic evaluation in PTPs with hemophilia B (Factor IX activity ≤ 2%). The efficacy evaluation included 105 PTPs [62 adults (18 to 65 years old), 18 adolescents (13 to 17 years old), and 25 children (1 to 12 years old)].
- •
- Adult/adolescent trial: The trial included 74 adolescent and adult subjects. There were two routine prophylaxis arms, with single-blind randomization to either 10 IU/kg or 40 IU/kg once-weekly for approximately 52 weeks, and an open-label on-demand treatment arm for approximately 28 weeks.
- •
- Surgery trial: The surgery trial included 13 adolescent and adult subjects who received one infusion of REBINYN 80 IU/kg on the day of surgery, and post-operatively received infusions of 40 IU/kg, at the investigator’s discretion, for up to 3 weeks after surgery.
- •
- Pediatric trial: The main phase of the pediatric trial included 25 PTPs (1 to 12 years old) in which subjects received routine prophylaxis with REBINYN 40 IU/kg once weekly for approximately 52 weeks until 50 EDs were reached.
Treatment of Bleeding Episodes
Previously Treated Patients
A total of 250 bleeding episodes were reported in 45 out of 69 PTPs receiving either REBINYN 40 IU/kg prophylaxis or on-demand treatment in the clinical program. Bleeding episodes were treated with REBINYN at 40 IU/kg for minor or moderate bleeds or 80 IU/kg for major bleeds, with additional doses of 40 IU/kg as needed. The median dose to treat a bleeding episode was 42 IU/kg.
An overall assessment of efficacy was performed by the subject (for home treatment) or the study site investigator (for treatment under medical supervision) using a 4-point scale of excellent, good, moderate, or poor. The overall success rate (defined as excellent or good) for treatment of bleeding episodes was 95% as shown in Table 9.
The success rate and dose needed for treatment of bleeding episodes were independent of the location of the bleeding. The success rate for treatment of bleeding episodes was also independent of whether the bleed was traumatic or spontaneous.
Table 9: Treatment of Bleeding Episodes in PTPs Receiving Either 40 IU/kg Prophylaxis or
On-Demand Treatment
|
Treatment |
Prophylaxis with 40 IU/kg |
On-Demand |
Total |
|
New Bleeding Episodes |
107 |
143 |
250 |
|
Efficacy assessment* | |||
|
Excellent or Good |
101 (95%) |
135 (95%) |
236 (95%) |
|
Moderate or Poor |
5 (5%) |
7 (5%) |
12 (5%) |
|
Number of injections to treat a bleeding episode | |||
|
1 injection |
100 (93%) |
120 (84%) |
220 (88%) |
|
2 injections |
5 (5%) |
20 (14%) |
25 (10%) |
|
>2 injections |
2 (2%) |
3 (2%) |
5 (2%) |
*Efficacy assessment was based on 248 evaluated bleeding episodes (data missing for two bleeding episodes). Efficacy was assessed according to a four-point scale using:
Excellent: Abrupt pain relief and/or clear improvement in objective signs of bleeding within 8 hours after a single injection; Good: Noticeable pain relief and/or improvement in signs of bleeding within 8 hours after a single injection;
Moderate: Probable or slight beneficial effect within the first 8 hours after the first injection but requiring more than one injection within 8 hours;
Poor: No improvement, or worsening of symptoms within 8 hours after the second of two injections.
Perioperative Management
In the surgery trial, the efficacy analysis of REBINYN in perioperative management included 13 surgical procedures of which 9 were major and performed in 13 previously treated adolescent and adult patients. The procedures included 9 orthopedic, 1 gastrointestinal and 3 in the oral cavity.
The hemostatic effect during surgery was evaluated on a four-point scale of excellent, good, moderate, or poor. The intraoperative hemostatic effect was rated as excellent or good for the 13 surgeries, for a success rate of 100%. A pre-operative dose of 80 IU/kg REBINYN was effective, and no subjects required additional doses on the day of surgery. The median number of additional 40 IU/kg doses in the post-operative period was 2.0 for Days 1 to 6, 1.5 for Days 7-13, and 3.0 for Days 1 to 13. The mean total consumption of REBINYN in the pre- and post-operative period was 241 IU/kg (range: 81 to 460 IU/kg). There was no unexpected postoperative bleeding.
Three additional major surgeries and 18 minor surgery procedures were evaluated in the extension trial for REBINYN in previously treated patients. The hemostatic effect during major and minor surgery was confirmed with a success rate of 100%.
Routine Prophylaxis
Adult/Adolescent PTP Trial
In the main phase of the adult/adolescent PTP trial, 29 subjects (13 to 65 years old) received REBINYN 40 IU/kg once
weekly for approximately 52 weeks. The annualized bleeding rate for these subjects in the main phase is presented in Table 10. Eighteen of 20 (90%) target joints per ISTH definition (≥ 3 spontaneous bleeds into a single joint within a consecutive 6-month period) reported in 13 subjects in the 40 IU/kg once weekly arm at baseline were considered resolved at the end of the main phase.
Table 10: Annualized Bleeding Rate (ABR) in the Adult/Adolescent PTP Trial (40 IU/kg Once Weekly Arm) –
Main Phase
|
Main Phase |
|||
|
Age of patient |
13-17 years N=9 |
18-65 years N=20 |
Overall ≥ 13 years N=29 |
|
Total ABR | |||
|
Poisson-estimated mean (95% CI) |
2.19 (0.73 ; 6.54) |
2.68 (1.34 ; 5.35) |
2.52 (1.40; 4.52) |
|
Median (Q1; Q3) |
1.93 (0.00 ; 4.01) |
1.03 (0.00 ; 4.01) |
1.04 (0.00; 4.01) |
|
ABR for spontaneous bleeds | |||
|
Poisson-estimated mean (95% CI) |
0.11 (0.00; 13.23) |
1.77 (0.77; 4.07) |
1.22 (0.46; 3.25) |
|
Median (Q1; Q3) |
0.00 (0.00; 0.00) |
0.00 (0.00; 1.51) |
0.00 (0.00; 0.99) |
|
ABR for traumatic bleeds | |||
|
Poisson-estimated mean (95% CI) |
2.08 (0.98; 4.42) |
0.91 (0.41; 2.02) |
1.29 (0.74; 2.25) |
|
Median (Q1; Q3) |
1.93 (0.00; 3.87) |
0.00 (0.00; 1.01) |
0.00 (0.00; 2.05) |
|
ABR for joint bleeds | |||
|
Poisson-estimated mean (95% CI) |
1.42 (0.36; 5.57) |
2.19 (1.02; 4.73) |
1.94 (0.97; 3.88) |
|
Median (Q1; Q3) |
0.97 (0.00; 2.17) |
0.51 (0.00; 2.04) |
0.97 (0.00; 2.07) |
CI = confidence interval; Q1 = first quartile; Q3 = third quartile.
Pediatric PTP Trial
In the main phase of the pediatric PTP trial, 25 subjects 0 to 12 years of age received routine prophylactic administration of REBINYN 40 IU/kg once weekly for 52 weeks. The patients were stratified into two age groups: 0 to 6 years and 7 to 12 years, with a least 10 subjects in each arm. Two target joints in 2 subjects in the 7 to 12 years age group at baseline were considered resolved during the main phase.
Table 11: Annualized Bleeding Rate (ABR) in the Pediatric PTP Trial - Main Phase
|
||
|
Age of patient |
|
|
|
Mean treatment period (years) |
|
|
|
Total ABR | ||
|
Poisson-estimated mean (95% CI) |
0.90 (0.43 ; 1.89) |
2.06 (1.31 ; 3.24) |
|
Median (Q1; Q3) |
0.00 (0.00 ; 1.98) |
2.00 (0.96 ; 3.00) |
|
ABR for spontaneous bleeds | ||
|
Poisson-estimated mean (95% CI) |
0.27 (0.06 ; 1.26) |
0.61 (0.24 ; 1.57) |
|
Median (Q1; Q3) |
0.00 (0.00 ; 0.00) |
0.00 (0.00 ; 0.96) |
|
ABR for traumatic bleeds | ||
|
Poisson-estimated mean (95% CI) |
0.63 (0.28 ; 1.43) |
1.14 (0.65 ; 2.01) |
|
Median (Q1; Q3) |
0.00 (0.00 ; 1.00) |
0.98 (0.00 ; 1.93) |
|
ABR for joint bleeds | ||
|
Poisson-estimated mean (95% CI) |
0.18 (0.04 ; 0.72) |
0.92 (0.52 ; 1.62) |
|
Median (Q1; Q3) |
0.00 (0.00 ; 0.00) |
0.96 (0.00 ; 1.93) |
CI = confidence interval; Q1 = first quartile; Q3 = third quartile.
16. How is Rebinyn supplied
How Supplied
- •
- REBINYN is supplied in packages comprised of a single-dose vial containing nominally 500, 1000, 2000, or 3000 IU of Factor IX potency; a MixPro® pre-filled diluent syringe containing 10 mM histidine solution (1.6 mg/mL), and a sterile vial adapter with 25 micrometer filter, which serves as a needleless reconstitution device.
- •
- The actual Factor IX potency in IU is stated on each REBINYN carton and vial.
Table 12: REBINYN Presentations
|
Presentation (Nominal Product Strength; IU) |
Cap Color Indicator |
Carton NDC Number |
Components |
|
500 |
Red |
NDC 0169 7905 01 |
|
|
1000 |
Green |
NDC 0169 7901 01 |
|
|
2000 |
Yellow |
NDC 0169 7902 01 |
|
|
3000 |
Dark Gray |
NDC 0169 7903 01 |
|
- •
- The REBINYN vials are made of glass, closed with a chlorobutyl rubber stopper (not made with natural rubber latex), and sealed with an aluminum cap.
- •
- The pre-filled diluent syringes are made of glass, with a siliconised bromobutyl rubber plunger (not made with rubber latex).
- •
- The closed vials and pre-filled diluent syringes are equipped with a tamper-evident snap-off cap which is made of polypropylene.
Storage and Handling
- •
- Store REBINYN in the original package in order to protect from light.
- •
- Store REBINYN under refrigeration at a temperature of 36°F-46°F (2°C – 8°C) for up to 24 months from the date of manufacture until the expiration date stated on the label.
- •
- REBINYN may be stored at room temperature not to exceed 86°F (30°C) for up to 6 months within the 24-month time period. Record the date when the product was removed from the refrigerator in the space provided on the outer carton. The total time of storage at room temperature should not exceed 6 months. Do not return the product to the refrigerator.
- •
- Do not use REBINYN after the end of the 6-month period at room temperature storage, or after the expiration date stated on the vial, whichever occurs earlier.
- •
- Do not freeze REBINYN.
- •
- Use REBINYN within 4 hours after reconstitution when stored at room temperature. Store the reconstituted product in the vial.
- •
- Discard any unused reconstituted product.
17. Patient Counseling Information
- •
- Advise patients to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
- •
- Inform patients of the early signs of hypersensitivity reactions including rash, hives, itching, facial swelling, tightness of the chest and wheezing. Advise patients to discontinue use of the product and contact their healthcare provider if these symptoms occur.
- •
- Advise patients to contact their healthcare provider for further treatment and/or assessment if they experience a lack of a clinical response to Factor IX therapy, as in some cases this may be a manifestation of an inhibitor.
- •
- Advise patients to contact their healthcare provider if they experience any thrombotic complications.
- •
- Advise patients to follow the recommendations regarding proper sharps disposal provided in the FDA-approved Instructions for Use.
Version: 4
License Number: 1261
REBINYN® and MixPro® are trademarks of Novo Nordisk A/S.
For Patent Information, refer to: http://novonordisk-us.com/patients/products/product-patents.html
Clave® and MicroClave® are registered trademarks of ICU Medical Inc.
InVision-Plus®, InVision-Plus CS®, Invision-Plus® Junior® are registered trademarks of RyMed Technologies, Inc.
Bionector® is a registered trademark of Vygon.
© 2022 Novo Nordisk
For information contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
1-844-REB-INYN
Manufactured by:
Novo Nordisk A/S
Novo Allé, DK-2880 Bagsvaerd
Patient Package Insert
Patient Product Information
REBINYN (reh-bē-NINE)
Coagulation Factor IX (Recombinant), GlycoPEGylated
Read the Patient Product Information and the Instructions For Use that come with REBINYN before you start taking this medicine and each time you get a refill, as there may be new information.
This Patient Product Information does not take the place of talking with your healthcare provider about your medical condition or treatment. If you have questions about REBINYN after reading this information, ask your healthcare provider.
What is the most important information I need to know about REBINYN?
Do not attempt to do an infusion yourself unless you have been taught how by your healthcare provider or hemophilia treatment center.
You must carefully follow your healthcare provider's instructions regarding the dose and schedule for infusing REBINYN so that your treatment will work best for you.
What is REBINYN?
REBINYN is an injectable medicine used to replace clotting Factor IX that is missing in patients with hemophilia B. Hemophilia B is an inherited bleeding disorder in all age groups that prevents blood from clotting normally.
REBINYN is used to treat, prevent, or reduce the frequency (number) of bleeding episodes in people with hemophilia B.
Your healthcare provider may give you REBINYN when you have surgery.
Who should not use REBINYN?
You should not use REBINYN if you
- •
- are allergic to Factor IX or any of the other ingredients of REBINYN
- •
- if you are allergic to hamster proteins
If you are not sure, talk to your healthcare provider before using this medicine.
Tell your healthcare provider if you are pregnant or nursing because REBINYN might not be right for you.
What should I tell my healthcare provider before I use REBINYN?
You should tell your healthcare provider if you
- •
- Have or have had any medical conditions.
- •
- Take any medicines, including non-prescription medicines and dietary supplements.
- •
- Are nursing. It is not known if REBINYN passes into breast milk or if it can harm your baby.
- •
- Are pregnant or planning to become pregnant. It is not known if REBINYN may harm your unborn baby.
- •
- Have been told that you have inhibitors to Factor IX (because REBINYN may not work for you).
How should I use REBINYN?
Treatment with REBINYN should be started by a healthcare provider who is experienced in the care of patients with hemophilia B.
REBINYN is given as an infusion into the vein.
You may infuse REBINYN at a hemophilia treatment center, at your healthcare provider's office or in your home. You should be trained on how to do infusions by your hemophilia treatment center or healthcare provider. Many people with hemophilia B learn to infuse the medicine by themselves or with the help of a family member.
Your healthcare provider will tell you how much REBINYN to use based on your weight, the severity of your hemophilia B, and where you are bleeding. Your dose will be calculated in international units, IU.
Call your healthcare provider right away if your bleeding does not stop after taking REBINYN.
If your bleeding is not adequately controlled, it could be due to the development of Factor IX inhibitors. This should be checked by your healthcare provider. You might need a higher dose of REBINYN or even a different product to control bleeding. Do not increase the total dose of REBINYN to control your bleeding without consulting your healthcare provider.
Use in children
REBINYN can be used in children. Your healthcare provider will decide the dose of REBINYN you will receive.
If you forget to use REBINYN
If you forget a dose, infuse the missed dose when you discover the mistake. Do not infuse a double dose to make up for a forgotten dose. Proceed with the next infusions as scheduled and speak to your healthcare provider if you have any questions or concerns.
If you stop using REBINYN
Do not stop using REBINYN without consulting your healthcare provider.
If you have any further questions on the use of this product, ask your healthcare provider.
What if I take too much REBINYN?
Always take REBINYN exactly as your healthcare provider has told you. You should check with your healthcare provider if you are not sure. If you infuse more REBINYN than recommended, tell your healthcare provider as soon as possible.
What are the possible side effects of REBINYN?
Common Side Effects Include:
- •
- infusion site reaction (bruising, bleeding, swelling, pain, or redness)
- •
- itching
- •
- rash
Your body can also make antibodies called “inhibitors” against Factor IX, including REBINYN, which may stop REBINYN from working properly. Your healthcare provider may need to test your blood for inhibitors from time to time.
You could have an allergic reaction to coagulation Factor IX products. Call your healthcare provider right away or get emergency treatment right away if you get, for example, any of the following signs of an allergic reaction: hives, chest tightness, wheezing, difficulty breathing, and/or swelling of the face.
You may be at an increased risk of forming blood clots in your body, especially if you have risk factors for developing blood clots. Call your healthcare provider if you have chest pain, difficulty breathing, leg tenderness or swelling.
These are not all of the possible side effects from REBINYN. Ask your healthcare provider for more information. You are encouraged to report side effects to FDA at 1-800-FDA-1088.
Tell your healthcare provider about any side effect that bothers you or that does not go away.
What are the REBINYN dosage strengths?
REBINYN comes in four different dosage strengths. The actual number of international units (IU) of Factor IX in the vial will be imprinted on the label and on the box. The four different strengths are as follows:
|
Cap Color Indicator |
Nominal Strength |
|
Red |
500 IU per vial |
|
Green |
1000 IU per vial |
|
Yellow |
2000 IU per vial |
|
Dark Gray |
3000 IU per vial |
Always check the actual dosage strength printed on the label to make sure you are using the strength prescribed by your healthcare provider.
How should I store REBINYN?
Prior to Reconstitution (mixing the dry powder in the vial with the diluent):
Store in original package in order to protect from light. Do not freeze REBINYN.
REBINYN vials can be stored in the refrigerator (36-46°F [2°C – 8°C]) for up to 24 months until the expiration date, or at room temperature (up to 86°F [30°C]) for a single period not more than 6 months.
If you choose to store REBINYN at room temperature:
- •
- Note the date that the product is removed from refrigeration on the box.
- •
- The total time of storage at room temperature should not be more than 6 months. Do not return the product to the refrigerator.
- •
- Do not use after 6 months from this date or the expiration date listed on the vial, whichever is earlier.
Do not use this medicine after the expiration date which is on the outer carton and the vial. The expiration date refers to the last day of that month.
After Reconstitution:
The reconstituted (the final product once the powder is mixed with the diluent) REBINYN should appear clear without visible particles.
The reconstituted REBINYN should be used immediately.
If you cannot use the reconstituted REBINYN immediately, it should be used within 4 hours when stored at or below 86ºF (30°C). Store the reconstituted product in the vial.
Keep this medicine out of the sight and out of reach of children.
What else should I know about REBINYN and hemophilia B?
Medicines are sometimes prescribed for purposes other than those listed here. Do not use REBINYN for a condition for which it is not prescribed. Do not share REBINYN with other people, even if they have the same symptoms that you have.
For more information about REBINYN, please call Novo Nordisk at 1-844-REB-INYN.
Revised: 08/2022
REBINYN® is a trademark of Novo Nordisk A/S.
For Patent Information, refer to:
http://novonordisk-us.com/products/product-patents.html
© 2022 Novo Nordisk
Manufactured by:
Novo Nordisk A/S
Novo Allé
DK-2880 Bagsværd, Denmark
For information about REBINYN contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
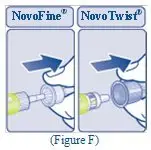
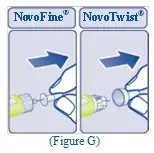
Instructions on how to use REBINYN® MixPro®
READ THESE INSTRUCTIONS CAREFULLY BEFORE USING REBINYN.
REBINYN is supplied as a powder. Before infusion (administration) it must be mixed (reconstituted) with the liquid diluent supplied in the syringe. The liquid diluent is a histidine solution. The mixed REBINYN must be infused into your vein (intravenous infusion). The equipment in this package is designed to mix and infuse REBINYN.
You will also need an infusion set (tubing and butterfly needle), sterile alcohol swabs, gauze pads, and bandages.
Don’t use the equipment without proper training from your doctor or nurse.
Always wash your hands and ensure that the area around you is clean.
When you prepare and infuse medication directly into the veins, it is important to use a clean and germ free (aseptic) technique. Improper technique can introduce germs that can infect the blood.
Don’t open the equipment until you are ready to use it.
Don’t use the equipment if it has been dropped, or if it is damaged. Use a new package instead.
Don’t use the equipment if it is expired. Use a new package instead. The expiration date is printed on the outer carton and on the vial, the vial adapter and the pre-filled syringe.
Don’t use the equipment if you suspect it is contaminated. Use a new package instead.
Don’t dispose of any of the items until after you have infused the mixed solution.
The equipment is for single use only.
Single-dose container. Discard unused portion.
Content
The package contains:
• Vial with REBINYN powder
• Vial adapter
• Pre-filled syringe with diluent
• Plunger rod (placed under the syringe)
1. Prepare the vial and the syringe
- •
- Take out the number of REBINYN® packages you need.
- •
- Check the expiry date.
- •
- Check the name, strength and color of the package, to make sure it contains the correct product.
- •
- Wash your hands and dry them properly using a clean towel or air dry.
- •
- Take the vial, the vial adapter and the pre-filled syringe out of the carton. Leave the plunger rod untouched in the carton.
- •
- Bring the vial and the pre-filled syringe to room temperature. You can do this by holding them in your hands until they feel as warm as your hands.
- •
- Remove the plastic cap from the vial. If the plastic cap is loose or missing, don’t use the vial.
- •
- Wipe the rubber stopper with a sterile alcohol swab and allow it to air dry for a few seconds before use to ensure that it is as germ free as possible.
- •
- Don’t touch the rubber stopper with your fingers as this can transfer germs.
2. Attach the vial adapter
- •
- Remove the protective paper from the vial adapter.
Don’t take the vial adapter out of the protective cap with your fingers. If you touch the spike on the vial adapter germs from your fingers can be transferred.
If the protective paper is not fully sealed or if it is broken, don’t use the vial adapter.
- •
- Place the vial on a flat and solid surface.
- •
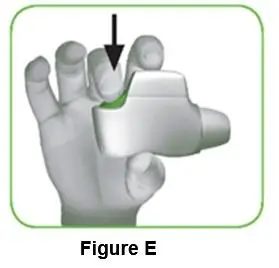
- Turn over the protective cap, and snap the vial adapter onto the vial.
- Once attached, don’t remove the vial adapter from the vial.
- •
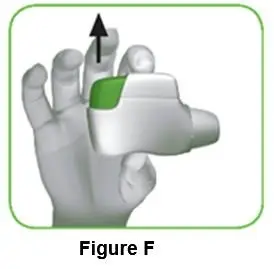
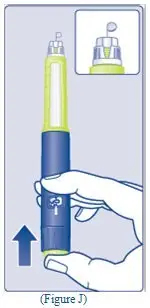
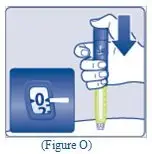
- Lightly squeeze the protective cap with your thumb and index finger as shown. Remove the protective cap from the vial adapter.
- Don’t lift the vial adapter from the vial when removing the protective cap.
3. Attach the plunger rod and the syringe
- •
- Grasp the plunger rod by the wide top end and take it out of the carton. Don’t touch the sides or the thread of the plunger rod. If you touch the sides or the thread germs from your fingers can be transferred.
- •
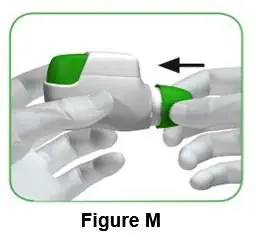
- Immediately connect the plunger rod to the syringe by turning it clockwise into the rubber plunger inside the pre-filled syringe until resistance is felt.
- •
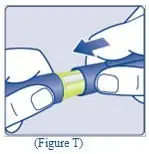
- Remove the syringe cap from the pre-filled syringe by bending it down until the perforation breaks.
- Don’t touch the syringe tip under the syringe cap. If you touch the syringe tip germs from your fingers can be transferred.
- If the syringe cap is loose or missing, don’t use the pre-filled syringe.
- •
- Screw the pre-filled syringe securely onto the vial adapter until resistance is felt.
4. Mix the powder with the diluent
- •
- Hold the pre-filled syringe slightly tilted with the vial pointing downwards.
- •
- Push the plunger rod to inject all the diluent into the vial.
- •
- Keep the plunger rod pressed down and swirl the vial gently until all the powder is dissolved.
- Don’t shake the vial as this will cause foaming.
- •
- Check the mixed solution. It must be clear and colorless. If you notice visible particles or discoloration, don’t use it. Use a new package instead.
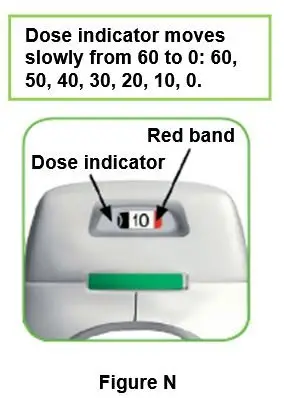
REBINYN is recommended to be used immediately after it is mixed.
If you cannot use the mixed REBINYN solution immediately, it should be used within 4 hours when stored at room temperature at or below 86°F (30°C). Store the reconstituted product in the vial.
Do not freeze mixed REBINYN solution or store it in syringes.
- Keep mixed REBINYN solution out of direct light.
- If your dose requires more than one vial, repeat step A to J with additional vials, vial adapters and pre-filled syringes until you have reached your required dose.
- •
- Keep the plunger rod pushed completely in.
- •
- Turn the syringe with the vial upside down.
- •
- Stop pushing the plunger rod and let it move back on its own while the mixed solution fills the syringe.
- •
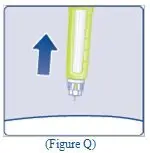
- Pull the plunger rod slightly downwards to draw the mixed solution into the syringe.
- •
- In case you only need part of the entire vial, use the scale on the syringe to see how much mixed solution you withdraw, as instructed by your doctor or nurse.
- •
- While holding the vial upside down, tap the syringe gently to let any air bubbles rise to the top.
- •
- Push the plunger rod slowly until all air bubbles are gone.
- •
- Unscrew the vial adapter with the vial.
Don’t touch the syringe tip. If you touch the syringe tip germs from your fingers can be transferred.
Caution: The pre-filled diluent syringe is made of glass with an internal tip diameter of 0.037 inches, and is compatible with a standard Luer-lock connector.
Some needleless connectors for intravenous catheters are incompatible with the glass diluent syringes (for example, certain connectors with an internal spike, such as Clave® /MicroClave®, InVision-Plus®, InVision-Plus CS®, Invision-Plus® Junior®, Bionector®).
The use of these needleless connectors can damage the connector and affect administration.
To administer REBINYN through incompatible needleless connectors, withdraw reconstituted product into a standard 10 mL sterile Luer-lock plastic syringe.
If you have encountered any problems with attaching the pre-filled histidine diluent syringe to any Luer‐lock compatible device, please contact Novo Nordisk at (844) 303-4448.
- 5.
- Infuse the mixed solution
REBINYN is now ready to infuse into your vein.
- •
- Do not mix REBINYN with any other intravenous infusions or medications.
- •
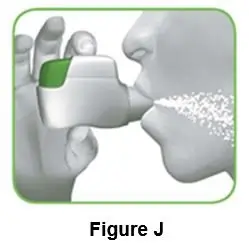
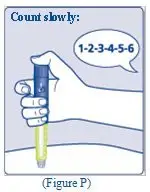
- Infuse the mixed solution slowly over 1 to 4 minutes as instructed by your doctor or nurse.
Infusing the solution via a central venous access device (CVAD) such as a central venous catheter or subcutaneous port:
- •
- Use a clean and germ free (aseptic) technique. Follow the instructions for proper use for your connector and central venous access device in consultation with your doctor or nurse.
- •
- Infusing into a CVAD may require using a sterile 10 mL plastic syringe for withdrawal of the mixed solution and infusion.
- •
- If necessary, use 0.9% Sodium Chloride Injection, USP to flush the CVAD line before or after REBINYN infusion.
The peel-off label found on the REBINYN vial can be used to record the lot number.
Disposal
- •
- After infusion, safely dispose of all unused REBINYN solution, the syringe with the infusion set, the vial with the vial adapter, and other waste materials in an appropriate container for throwing away medical waste.
- Don’t throw it out with the ordinary household trash.
Don’t disassemble the vial and vial adapter before disposal.
Don’t reuse the equipment.
Important information
Contact your healthcare provider or local hemophilia treatment center if you experience any problems.
For full Prescribing Information please read the other insert included in this package.
REBINYN® and MixPro® are trademarks of Novo Nordisk A/S.
For Patent Information, refer to: http://novonordisk-us.com/patients/products/product-patents.html
Clave® and MicroClave® are registered trademarks of ICU Medical Inc.
InVision-Plus®, InVision-Plus CS®, Invision-Plus®Junior® are registered trademarks of RyMed Technologies, Inc.
Bionector is a registered trademark of Vygon
© 2020 Novo Nordisk
Manufactured by:
Novo Nordisk A/S
DK-2880 Bagsvaerd, Denmark
For information about REBINYN contact:
Novo Nordisk Inc.
800 Scudders Mill Road
Plainsboro, NJ 08536, USA
License No: 1261
- Revised: 06/2020
PRINCIPAL DISPLAY PANEL
NDC 0169 7905 01 List: 790501 500 IU Range
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
500 IU
Intravenous use, after reconstitution.
Single-dose. Discard unused portion.
Contains no preservatives.
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe
Principal Display Panel
NDC 0169 7901 01 List: 790101 1000 IU Range
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
1000 IU
Intravenous use, after reconstitution.
Single-dose. Discard unused portion.
Contains no preservatives.
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe
Principal Display Panel
NDC 0169 7902 01 List: 790201 2000 IU Range
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
2000 IU
Intravenous use, after reconstitution.
Single-dose. Discard unused portion.
Contains no preservatives.
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe
NDC 0169 7955 11
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
500 IU
Store in a refrigerator 36°F - 46°F (2°C-8°C)
Do not freeze
Rx Only
Reconstitution with
4 mL histidine
diluent only
NDC 0169 7911 11
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
1000 IU
Store in a refrigerator 36°F - 46°F (2°C-8°C)
Do not freeze
Rx Only
Reconstitution with
4 mL histidine
diluent only
NDC 0169 7922 11
REBINYN®
(Coagulation Factor IX (Recombinant), GlycoPEGylated)
2000 IU
Store in a refrigerator 36°F - 46°F (2°C-8°C)
Do not freeze
Rx Only
Reconstitution with
4 mL histidine
diluent only
NDC 0169 7009 98
Histidine (10 mM Solution) 4 mL
For reconstitution of Tradename
Store in a refrigerator 36°F to 46°F (2°C to 8°C)
Do not freeze
Rx Only
Single Use Only
PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
NDC 0169 7903 01
List: 790301
- REBINYN®3000 IU Range
Coagulation Factor IX (Recombinant), GlycoPEGylated
3000 IU
Intravenous use, after reconstitution.
Single-dose. Discard unused portion.
Contains no preservatives.
Rx Only
Includes MixPro®
a vial adapter and pre-filled diluent syringe
NDC 0169 7933 11
- REBINYN®3000 IU Range
Coagulation Factor IX (Recombinant), GlycoPEGylated
Store in a refrigerator
36°F - 46°F (2°C-8°C)
Do not freeze
Rx Only
Reconstitution with
4 mL histidine
diluent only
| REBINYN
(coagulation factor ix (recombinant), glycopegylated) kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| REBINYN
(coagulation factor ix (recombinant), glycopegylated) kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| REBINYN
(coagulation factor ix (recombinant), glycopegylated) kit |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| REBINYN
(coagulation factor ix (recombinant), glycopegylated) kit |
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
|
|||||||||||||||
| Labeler - Novo Nordisk (622920320) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novo Nordisk A/S - Hax | 306711800 | API MANUFACTURE(0169-7905, 0169-7955, 0169-7009, 0169-7901, 0169-7911, 0169-7009, 0169-7902, 0169-7922, 0169-7009, 0169-7903, 0169-7933, 0169-7009) , MANUFACTURE(0169-7905, 0169-7955, 0169-7009, 0169-7901, 0169-7911, 0169-7009, 0169-7902, 0169-7922, 0169-7009, 0169-7903, 0169-7933, 0169-7009) | |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Novo Nordisk A/S - 25a-B | 309477891 | API MANUFACTURE(0169-7905, 0169-7955, 0169-7009, 0169-7901, 0169-7911, 0169-7009, 0169-7902, 0169-7922, 0169-7009, 0169-7903, 0169-7933, 0169-7009) | |