Drug Detail:Rhofade (Oxymetazoline topical [ ox-ee-me-taz-o-leen-top-i-kal ])
Drug Class: Topical anti-rosacea agents
Highlights of Prescribing Information
RHOFADE® (oxymetazoline hydrochloride) cream, 1%, for topical use
Initial U.S. Approval: 1964
Indications and Usage for Rhofade Cream
Rhofade cream is an alpha1A adrenoceptor agonist indicated for the topical treatment of persistent facial erythema associated with rosacea in adults. (1)
Rhofade Cream Dosage and Administration
- Not for oral, ophthalmic, or intravaginal use. (2)
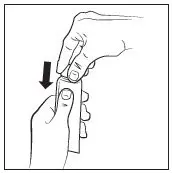
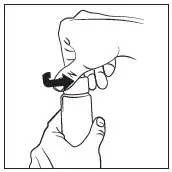
- Prime pump bottle before initial use and discard product from first three pumps. (2)
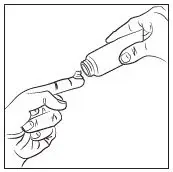
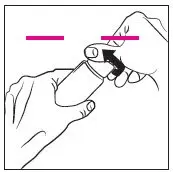
- Apply a pea-sized amount once daily in a thin layer to cover the entire face (forehead, nose, each cheek, and chin) avoiding the eyes and lips. (2)
- Wash hands after application. (2)
Dosage Forms and Strengths
Cream, 1%. Each gram of cream contains 10 mg (1%) oxymetazoline hydrochloride, equivalent to 8.8 mg (0.88%) of oxymetazoline free base. (3)
Contraindications
- None. (4)
Warnings and Precautions
- Alpha-adrenergic agonists as a class may impact blood pressure. Advise patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension or hypotension to seek medical care if their condition worsens. (5.1)
- Use with caution in patients with cerebral or coronary insufficiency, Raynaud's phenomenon, thromboangiitis obliterans, scleroderma, or Sjögren's syndrome and advise patients to seek medical care if signs and symptoms of potentiation of vascular insufficiency develop. (5.2)
- Advise patients to seek immediate medical care if signs and symptoms of acute narrow-angle glaucoma develop. (5.3)
Adverse Reactions/Side Effects
Most common adverse reactions (incidence ≥ 1%) are application site dermatitis, worsening inflammatory lesions of rosacea, application site pruritis, application site erythema, and application site pain. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact EPI Health, LLC at 1-800-499-4468 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 11/2019
Related/similar drugs
doxycycline, metronidazole topical, ivermectin topical, brimonidine topical, minocycline topical, VibramycinFull Prescribing Information
1. Indications and Usage for Rhofade Cream
RHOFADE® (oxymetazoline hydrochloride) cream, 1% is indicated for the topical treatment of persistent facial erythema associated with rosacea in adults.
2. Rhofade Cream Dosage and Administration
For topical use only. RHOFADE cream is not for oral, ophthalmic, or intravaginal use.
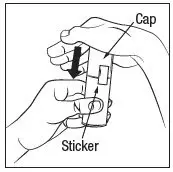
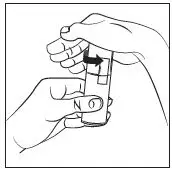
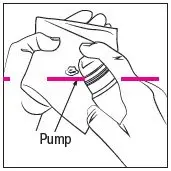
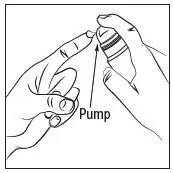
Prime the RHOFADE cream pump before using for the first time. To do so, with the pump in the upright position, repeatedly depress the actuator until cream is dispensed and then pump three times. Discard the cream from priming actuations. It is only necessary to prime the pump before the first dose.
RHOFADE cream tubes do not require priming.
Apply a pea-sized amount of RHOFADE cream, once daily in a thin layer to cover the entire face (forehead, nose, each cheek, and chin) avoiding the eyes and lips. Wash hands immediately after applying RHOFADE cream.
3. Dosage Forms and Strengths
RHOFADE® (oxymetazoline hydrochloride) cream, 1% is a white to off-white cream. Each gram of cream contains 10 mg (1%) oxymetazoline hydrochloride, equivalent to 8.8 mg (0.88%) of oxymetazoline free base.
5. Warnings and Precautions
5.1 Potential Impacts on Cardiovascular Disease
Alpha-adrenergic agonists may impact blood pressure. RHOFADE cream should be used with caution in patients with severe or unstable or uncontrolled cardiovascular disease, orthostatic hypotension, and uncontrolled hypertension or hypotension. Advise patients with cardiovascular disease, orthostatic hypotension, and/or uncontrolled hypertension/hypotension to seek immediate medical care if their condition worsens.
5.2 Potentiation of Vascular Insufficiency
RHOFADE cream should be used with caution in patients with cerebral or coronary insufficiency, Raynaud's phenomenon, thromboangiitis obliterans, scleroderma, or Sjögren's syndrome. Advise patients to seek immediate medical care if signs and symptoms of potentiation of vascular insufficiency develop.
6. Adverse Reactions/Side Effects
6.1 Clinical Studies Experience
Because clinical trials are conducted under varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
A total of 489 subjects with persistent facial erythema associated with rosacea were treated with RHOFADE cream once daily for 4 weeks in 3 controlled clinical trials. An additional 440 subjects with persistent facial erythema associated with rosacea were also treated with RHOFADE cream once daily for up to one year in a long-term (open-label) clinical trial. Adverse reactions that occurred in at least 1% of subjects treated with RHOFADE cream through 4 weeks of treatment are presented in Table 1 below.
| Adverse Reaction | Pooled Controlled Clinical Trials | |
|---|---|---|
| RHOFADE Cream (N = 489) | Vehicle Cream (N = 483) |
|
| Application site dermatitis | 9 (2%) | 0 |
| Worsening inflammatory lesions of rosacea | 7 (1%) | 1 (<1%) |
| Application site pruritus | 5 (1%) | 4 (1%) |
| Application site erythema | 5 (1%) | 2 (<1%) |
| Application site pain | 4 (1%) | 1 (<1%) |
In the long-term (open-label) clinical trial, the rates of adverse reactions over a one-year treatment period were as follows: worsening inflammatory lesions of rosacea (3%), application site dermatitis (3%), application site pruritis (2%), application site pain (2%), and application site erythema (2%). Subjects with persistent erythema along with inflammatory lesions were allowed to use additional therapy for the inflammatory lesions of rosacea.
7. Drug Interactions
7.1 Anti-hypertensives/Cardiac Glycosides
Alpha-adrenergic agonists, as a class, may impact blood pressure. Caution in using drugs such as beta-blockers, anti-hypertensives and/or cardiac glycosides is advised.
Caution should also be exercised in patients receiving alpha1 adrenergic receptor antagonists such as in the treatment of cardiovascular disease, benign prostatic hypertrophy, or Raynaud's disease.
8. Use In Specific Populations
8.2 Lactation
No clinical data are available to assess the effects of oxymetazoline on the quantity or rate of breastmilk production, or to establish the level of oxymetazoline present in human breastmilk post-dose. Oxymetazoline was detected in the milk of lactating rats. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for RHOFADE cream and any potential adverse effects on the breastfed child from RHOFADE cream or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of RHOFADE cream have not been established in pediatric patients below the age of 18 years.
8.5 Geriatric Use
One hundred and ninety-three subjects aged 65 years and older received treatment with RHOFADE cream (n = 135) or vehicle (n = 58) in clinical trials. No overall differences in safety or effectiveness were observed between subjects ≥ 65 years of age and younger subjects, based on available data. Clinical studies of RHOFADE cream did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects.
10. Overdosage
RHOFADE cream is not for oral use. If oral ingestion occurs, seek medical advice. Monitor patient closely and administer appropriate supportive measures as necessary. Accidental ingestion of topical solutions (nasal sprays) containing imidazoline derivatives (e.g., oxymetazoline) in children has resulted in serious adverse events requiring hospitalization, including nausea, vomiting, lethargy, tachycardia, decreased respiration, bradycardia, hypotension, hypertension, sedation, somnolence, mydriasis, stupor, hypothermia, drooling, and coma. Keep RHOFADE cream out of reach of children.
11. Rhofade Cream Description
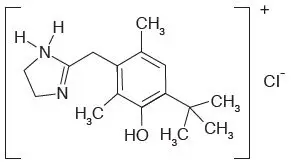
RHOFADE® (oxymetazoline hydrochloride) cream, 1% contains oxymetazoline hydrochloride, an alpha1A adrenoceptor agonist. RHOFADE is a white to off-white cream. It has a chemical name of 3-[(4,5-Dihydro1H-imidazol-2-yl)methyl]-6-(1,1-dimethylethyl)-2,4-dimethyl-phenol hydrochloride and a molecular weight of 296.8. It is freely soluble in water and ethanol and has a partition coefficient of 0.1 in 1-octanol/water. The molecular formula of oxymetazoline HCl is C16H25ClN2O and its structural formula is:
Each gram of RHOFADE® (oxymetazoline hydrochloride) cream contains 10 mg (1%) oxymetazoline hydrochloride, equivalent to 8.8 mg (0.88%) of oxymetazoline free base. The cream contains the following inactive ingredients: sodium citrate dihydrate, citric acid anhydrous, disodium edetate dihydrate, butylated hydroxytoluene, anhydrous lanolin, medium chain triglycerides, diisopropyl adipate, oleyl alcohol, polyethylene glycol 300, PEG-6 stearate, glycol stearate, PEG-32 stearate, cetostearyl alcohol, ceteareth-6, stearyl alcohol, ceteareth-25, methylparaben, propylparaben, phenoxyethanol, and purified water.
12. Rhofade Cream - Clinical Pharmacology
12.1 Mechanism of Action
Oxymetazoline is an alpha1A adrenoceptor agonist. Oxymetazoline acts as a vasoconstrictor.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Oxymetazoline hydrochloride was not associated with an increased incidence of neoplastic or proliferative changes in transgenic mice given oral doses of 0.5, 1.0, or 2.5 mg/kg/day oxymetazoline hydrochloride for 6 months.
Oxymetazoline hydrochloride revealed no evidence of mutagenic or clastogenic potential based on the results of two in vitro genotoxicity tests (Ames assay and human lymphocyte chromosomal aberration assay) and one in vivo genotoxicity test (mouse micronucleus assay).
Effects on fertility and early embryonic development were evaluated in rats following oral administration of 0.05, 0.1, or 0.2 mg/kg/day oxymetazoline hydrochloride prior to and during mating and through early pregnancy. Decreased number of corpora lutea and increased post-implantation losses were noted at 0.2 mg/kg/day oxymetazoline hydrochloride (3 times the MRHD on an AUC comparison basis). However, no treatment related effects on fertility or mating parameters were noted at 0.2 mg/kg/day oxymetazoline hydrochloride (3 times the MRHD on an AUC comparison basis).
14. Clinical Studies
RHOFADE cream was evaluated for the treatment of persistent erythema associated with rosacea in two identical, randomized, double-blind, vehicle-controlled, parallel-group clinical trials. The trials enrolled 885 subjects aged 18 years and older. Overall, 90% of subjects were Caucasian and 79% were female. Subjects applied either RHOFADE cream or vehicle once daily for 29 days.
Disease severity was graded by the clinician using a 5-point clinician erythema assessment (CEA) scale and by the subject on a similar 5-point subject self-assessment (SSA) scale, on which subjects scored either "moderate" or "severe" on both scales.
CEA and SSA were measured over a 12-hour period at equally-spaced timepoints (hours 3, 6, 9, and 12) post dose on Days 1, 15, and 29. The primary efficacy endpoint was defined as the proportion of subjects with at least a 2-grade reduction in erythema (improvement) from baseline (pre-dose on Day 1) on both the CEA and SSA measured at hours 3, 6, 9, and 12 on Day 29. The results from both trials on the composite endpoint for Day 29 are presented in Table 2.
| Timepoint (Hour) | Trial 1 | Trial 2 | ||
|---|---|---|---|---|
| RHOFADE Cream (N=222) | Vehicle Cream (N=218) | RHOFADE Cream (N = 224) | Vehicle Cream (N=221) |
|
|
||||
| 3 | 12% | 6% | 14% | 7% |
| 6 | 16% | 8% | 13% | 5% |
| 9 | 18% | 6% | 16% | 9% |
| 12 | 15% | 6% | 12% | 6% |
16. How is Rhofade Cream supplied
RHOFADE® (oxymetazoline hydrochloride) cream, 1%, is a white to off-white cream. The product is available in a laminated tube and an airless pump polypropylene bottle in the following packaging configurations, each with a child-resistant closure:
| NDC 71403-003-30 | 30 gram tube |
| NDC 71403-003-45 | 45 gram tube |
| NDC 71403-003-60 | 60 gram tube |
| NDC 71403-003-35 | 30 gram pump |
| NDC 71403-003-65 | 60 gram pump |
17. Patient Counseling Information
Advise the patient and/or caregiver to read the FDA-approved patient labeling (Patient Information and Instructions for Use).
| RHOFADE
oxymetazoline hydrochloride cream |
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||
| Labeler - EPI Health, LLC (080638894) |
| Registrant - DPT Laboratories, Ltd. (832224526) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| DPT Laboratories, Ltd. | 832224526 | MANUFACTURE(71403-003) | |