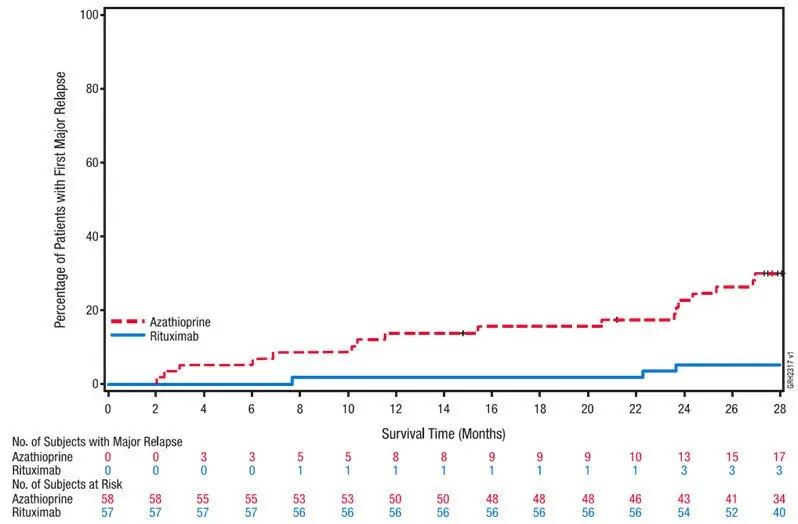
Drug Detail:Riabni (Rituximab [ ri-tux-i-mab ])
Drug Class: CD20 monoclonal antibodies
Highlights of Prescribing Information
RIABNI™ (rituximab-arrx) injection, for intravenous use
Initial U.S. Approval: 2020
RIABNI (rituximab-arrx) is biosimilar* to RITUXAN (rituximab)
WARNING: FATAL INFUSION-RELATED REACTIONS, SEVERE MUCOCUTANEOUS REACTIONS, HEPATITIS B VIRUS REACTIVATION and PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
See full prescribing information for complete boxed warning.
- Fatal infusion-related reactions within 24 hours of rituximab infusion; approximately 80% of fatal reactions occurred with first infusion. Monitor patients and discontinue RIABNI infusion for severe reactions (5.1).
- Severe mucocutaneous reactions, some with fatal outcomes (5.2).
- Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure, and death (5.3).
- Progressive multifocal leukoencephalopathy (PML) resulting in death (5.4).
Recent Major Changes
| Indications and Usage, Rheumatoid Arthritis (RA) (1.3) | 6/2022 |
| Dosage and Administration, Recommended Dose for Rheumatoid Arthritis (RA) (2.5) | 6/2022 |
| Dosage and Administration, Administration and Storage (2.8) | 6/2022 |
| Warnings and Precautions (5.10, 5.12, 5.13) | 6/2022 |
Indications and Usage for Riabni
RIABNI is a CD20-directed cytolytic antibody indicated for the treatment of:
- Adult patients with non-Hodgkin's Lymphoma (NHL) (1.1).
- Relapsed or refractory, low grade or follicular, CD20-positive B-cell NHL as a single agent.
- Previously untreated follicular, CD20-positive, B-cell NHL in combination with first line chemotherapy and, in patients achieving a complete or partial response to a rituximab product in combination with chemotherapy, as single-agent maintenance therapy.
- Non-progressing (including stable disease), low-grade, CD20-positive, B-cell NHL as a single agent after first-line cyclophosphamide, vincristine, and prednisone (CVP) chemotherapy.
- Previously untreated diffuse large B-cell, CD20-positive NHL in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or other anthracycline-based chemotherapy regimens.
- Adult patients with Chronic Lymphocytic Leukemia (CLL) (1.2).
- Previously untreated and previously treated CD20-positive CLL in combination with fludarabine and cyclophosphamide (FC).
- Rheumatoid Arthritis (RA) in combination with methotrexate in adult patients with moderately-to severely-active RA who have inadequate response to one or more TNF antagonist therapies (1.3).
- Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA) in adult patients in combination with glucocorticoids (1.3).
Riabni Dosage and Administration
- Administer only as an intravenous infusion (2.1).
- Do not administer as an intravenous push or bolus (2.1).
- RIABNI should only be administered by a healthcare professional with appropriate medical support to manage severe infusion-related reactions that can be fatal if they occur (2.1).
- The dose for adult B-cell NHL is 375 mg/m2 (2.2).
- The dose for CLL is 375 mg/m2 in the first cycle and 500 mg/m2 in cycles 2–6, in combination with FC, administered every 28 days (2.3).
- The dose as a component of Zevalin® (ibritumomab tiuxetan) Therapeutic Regimen is 250 mg/m2 (2.4).
- The dose for RA in combination with methotrexate is two-1,000 mg intravenous infusions separated by 2 weeks (one course) every 24 weeks or based on clinical evaluation, but not sooner than every 16 weeks. Methylprednisolone 100 mg intravenous or equivalent glucocorticoid is recommended 30 minutes prior to each infusion (2.5).
- The induction dose for adult patients with active GPA and MPA in combination with glucocorticoids is 375 mg/m2 once weekly for 4 weeks. The follow up dose for adult patients with GPA and MPA who have achieved disease control with induction treatment, in combination with glucocorticoids is two 500 mg intravenous infusions separated by two weeks, followed by a 500 mg intravenous infusion every 6 months thereafter based on clinical evaluation (2.6).
Dosage Forms and Strengths
- Injection: 100 mg/10 mL (10 mg/mL) and 500 mg/50 mL (10 mg/mL) solution in single-dose vials (3)
Contraindications
None (4)
Warnings and Precautions
- Tumor lysis syndrome: Administer aggressive intravenous hydration, anti-hyperuricemic agents, monitor renal function (5.5).
- Infections: Withhold RIABNI and institute appropriate anti-infective therapy (5.6).
- Cardiac adverse reactions: Discontinue infusions in case of serious or life-threatening events (5.7).
- Renal toxicity: Discontinue in patients with rising serum creatinine or oliguria (5.8).
- Bowel obstruction and perforation: Consider and evaluate for abdominal pain, vomiting, or related symptoms (5.9).
- Immunizations: Live virus vaccinations prior to or during RIABNI treatment is not recommended (5.10).
- Embryo-Fetal toxicity: Can cause fetal harm. Advise females of reproductive potential of the potential risk to a fetus and use of effective contraception (5.11).
Adverse Reactions/Side Effects
Most common adverse reactions in clinical trials were:
- NHL (greater than or equal to 25%): infusion-related reactions, fever, lymphopenia, chills, infection and asthenia (6.1).
- CLL (greater than or equal to 25%): infusion-related reactions and neutropenia (6.1).
- RA (greater than or equal to 10%): upper respiratory tract infection, nasopharyngitis, urinary tract infection, and bronchitis (other important adverse reactions include infusion-related reactions, serious infections, and cardiovascular events) (6.2).
- GPA and MPA (greater than or equal to 15%): infections, nausea, diarrhea, headache, muscle spasms, anemia, peripheral edema, infusion-related reactions (6.2).
To report SUSPECTED ADVERSE REACTIONS, contact Amgen, Inc. at 1-800-77-AMGEN (1-800-772-6436) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
Renal toxicity when used in combination with cisplatin (5.8).
Use In Specific Populations
- Lactation: Advise not to breastfeed (8.2).
- Geriatric Use: In CLL patients older than 70 years of age, exploratory analyses suggest no benefit with the addition of rituximab to FC (8.5).
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
- *
- Biosimilar means that the biological product is approved based on data demonstrating that it is highly similar to an FDA-approved biological product, known as a reference product, and that there are no clinically meaningful differences between the biosimilar product and the reference product. Biosimilarity of RIABNI has been demonstrated for the condition(s) of use (e.g., indication(s), dosing regimen(s), strength(s), dosage form(s) and route(s) of administration) described in its Full Prescribing Information.
Revised: 6/2022
Full Prescribing Information
WARNING: FATAL INFUSION-RELATED REACTIONS, SEVERE MUCOCUTANEOUS REACTIONS, HEPATITIS B VIRUS REACTIVATION and PROGRESSIVE MULTIFOCAL LEUKOENCEPHALOPATHY
1. Indications and Usage for Riabni
1.1 Non-Hodgkin's Lymphoma (NHL)
RIABNI is indicated for the treatment of adult patients with:
- Relapsed or refractory, low-grade or follicular, CD20-positive, B-cell NHL as a single agent.
- Previously untreated follicular, CD20-positive, B-cell NHL in combination with first line chemotherapy and, in patients achieving a complete or partial response to a rituximab product in combination with chemotherapy, as single-agent maintenance therapy.
- Non-progressing (including stable disease), low-grade, CD20-positive, B-cell NHL as a single agent after first-line cyclophosphamide, vincristine, and prednisone (CVP) chemotherapy.
- Previously untreated diffuse large B-cell, CD20-positive NHL in combination with cyclophosphamide, doxorubicin, vincristine, prednisone (CHOP) or other anthracycline-based chemotherapy regimens.
1.2 Chronic Lymphocytic Leukemia (CLL)
RIABNI, in combination with fludarabine and cyclophosphamide (FC), is indicated for the treatment of adult patients with previously untreated and previously treated CD20-positive CLL.
1.3 Rheumatoid Arthritis (RA)
RIABNI, in combination with methotrexate, is indicated for the treatment of adult patients with moderately- to severely- active rheumatoid arthritis who have had an inadequate response to one or more TNF antagonist therapies.
1.4 Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
RIABNI, in combination with glucocorticoids, is indicated for the treatment of adult patients with Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA).
2. Riabni Dosage and Administration
2.1 Important Dosing Information
Administer only as an intravenous infusion [see Dosage and Administration (2.7)]. Do not administer as an intravenous push or bolus. RIABNI should only be administered by a healthcare professional with appropriate medical support to manage severe infusion-related reactions that can be fatal if they occur [see Warnings and Precautions (5.1)].
Premedicate before each infusion [see Dosage and Administration (2.7)].
2.2 Recommended Dose for Non-Hodgkin's Lymphoma (NHL)
The recommended dose is 375 mg/m2 as an intravenous infusion according to the following schedules:
-
Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
Administer once weekly for 4 or 8 doses. -
Retreatment for Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
Administer once weekly for 4 doses. -
Previously Untreated, Follicular, CD20-Positive, B-Cell NHL
Administer on Day 1 of each cycle of chemotherapy, for up to 8 doses. In patients with complete or partial response, initiate RIABNI maintenance eight weeks following completion of a rituximab product in combination with chemotherapy. Administer RIABNI as a single-agent every 8 weeks for 12 doses. -
Non-progressing, Low-Grade, CD20-Positive, B-Cell NHL, after first-line CVP chemotherapy
Following completion of 6–8 cycles of CVP chemotherapy, administer once weekly for 4 doses at 6-month intervals to a maximum of 16 doses. -
Diffuse Large B-Cell NHL
Administer on Day 1 of each cycle of chemotherapy for up to 8 infusions.
2.3 Recommended Dose for Chronic Lymphocytic Leukemia (CLL)
The recommended dose is 375 mg/m2 the day prior to the initiation of FC chemotherapy, then 500 mg/m2 on Day 1 of cycles 2–6 (every 28 days).
2.4 Recommended Dose as a Component of Zevalin® for Treatment of NHL
When used as part of the Zevalin therapeutic regimen, infuse 250 mg/m2 in accordance with the Zevalin package insert. Refer to the Zevalin package insert for full prescribing information regarding the Zevalin therapeutic regimen.
2.5 Recommended Dose for Rheumatoid Arthritis (RA)
- Administer RIABNI as two-1,000 mg intravenous infusions separated by 2 weeks.
- Glucocorticoids administered as methylprednisolone 100 mg intravenous or its equivalent 30 minutes prior to each infusion are recommended to reduce the incidence and severity of infusion-related reactions.
- Subsequent courses should be administered every 24 weeks or based on clinical evaluation, but not sooner than every 16 weeks.
- RIABNI is given in combination with methotrexate.
2.6 Recommended Dose for Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
2.7 Recommended Dose for Premedication and Prophylactic Medications
Premedicate with acetaminophen and an antihistamine before each infusion of RIABNI. For adult patients administered RIABNI according to the 90-minute infusion rate, the glucocorticoid component of their chemotherapy regimen should be administered prior to infusion [see Clinical Studies (14.4)].
For RA, GPA, and MPA patients, methylprednisolone 100 mg intravenously or its equivalent is recommended 30 minutes prior to each infusion. Provide prophylaxis treatment for Pneumocystis jirovecii pneumonia (PCP) and herpes virus infections for patients with CLL during treatment and for up to 12 months following treatment as appropriate [see Warnings and Precautions (5.6)].
PCP prophylaxis is also recommended for patients with GPA and MPA during treatment and for at least 6 months following the last RIABNI infusion.
2.8 Administration and Storage
Use appropriate aseptic technique. Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. RIABNI should be a clear to slightly opalescent, colorless to slightly yellow liquid. Do not use vial if particulates or discoloration is present.
3. Dosage Forms and Strengths
Injection: 100 mg/10 mL (10 mg/mL) and 500 mg/50 mL (10 mg/mL) as a clear to slightly opalescent, colorless to slightly yellow solution in a single-dose vial.
5. Warnings and Precautions
5.1 Infusion-Related Reactions
Rituximab products can cause severe, including fatal, infusion-related reactions. Severe reactions typically occurred during the first infusion with time to onset of 30–120 minutes. Rituximab product-induced infusion-related reactions and sequelae include urticaria, hypotension, angioedema, hypoxia, bronchospasm, pulmonary infiltrates, acute respiratory distress syndrome, myocardial infarction, ventricular fibrillation, cardiogenic shock, anaphylactoid events, or death.
Premedicate patients with an antihistamine and acetaminophen prior to dosing. For RA, GPA and MPA patients, methylprednisolone 100 mg intravenously or its equivalent is recommended 30 minutes prior to each infusion. Institute medical management (e.g., glucocorticoids, epinephrine, bronchodilators, or oxygen) for infusion-related reactions as needed. Depending on the severity of the infusion-related reaction and the required interventions, temporarily or permanently discontinue RIABNI. Resume infusion at a minimum 50% reduction in rate after symptoms have resolved. Closely monitor the following patients: those with pre-existing cardiac or pulmonary conditions, those who experienced prior cardiopulmonary adverse reactions, and those with high numbers of circulating malignant cells (greater than or equal to 25,000/mm3) [see Warnings and Precautions (5.7), Adverse Reactions (6.1)].
5.2 Severe Mucocutaneous Reactions
Mucocutaneous reactions, some with fatal outcome, can occur in patients treated with rituximab products. These reactions include paraneoplastic pemphigus, Stevens-Johnson syndrome, lichenoid dermatitis, vesiculobullous dermatitis, and toxic epidermal necrolysis. The onset of these reactions has been variable and includes reports with onset on the first day of rituximab exposure. Discontinue RIABNI in patients who experience a severe mucocutaneous reaction. The safety of re-administration of rituximab products to patients with severe mucocutaneous reactions has not been determined.
5.3 Hepatitis B Virus (HBV) Reactivation
Hepatitis B virus (HBV) reactivation, in some cases resulting in fulminant hepatitis, hepatic failure and death, can occur in patients treated with drugs classified as CD20-directed cytolytic antibodies, including rituximab products. Cases have been reported in patients who are hepatitis B surface antigen (HBsAg) positive and also in patients who are HBsAg negative but are hepatitis B core antibody (anti-HBc) positive. Reactivation also has occurred in patients who appear to have resolved hepatitis B infection (i.e., HBsAg negative, anti-HBc positive and hepatitis B surface antibody [anti-HBs] positive).
HBV reactivation is defined as an abrupt increase in HBV replication manifesting as a rapid increase in serum HBV DNA levels or detection of HBsAg in a person who was previously HBsAg negative and anti-HBc positive. Reactivation of HBV replication is often followed by hepatitis, i.e., increase in transaminase levels. In severe cases increase in bilirubin levels, liver failure, and death can occur.
Screen all patients for HBV infection by measuring HBsAg and anti-HBc before initiating treatment with RIABNI. For patients who show evidence of prior hepatitis B infection (HBsAg positive [regardless of antibody status] or HBsAg negative but anti-HBc positive), consult with physicians with expertise in managing hepatitis B regarding monitoring and consideration for HBV antiviral therapy before and/or during RIABNI treatment.
Monitor patients with evidence of current or prior HBV infection for clinical and laboratory signs of hepatitis or HBV reactivation during and for several months following RIABNI therapy. HBV reactivation has been reported up to 24 months following completion of rituximab therapy.
In patients who develop reactivation of HBV while on RIABNI, immediately discontinue RIABNI and any concomitant chemotherapy, and institute appropriate treatment. Insufficient data exist regarding the safety of resuming RIABNI treatment in patients who develop HBV reactivation. Resumption of RIABNI treatment in patients whose HBV reactivation resolves should be discussed with physicians with expertise in managing HBV.
5.4 Progressive Multifocal Leukoencephalopathy (PML)
JC virus infection resulting in PML and death can occur in rituximab product-treated patients with hematologic malignancies or with autoimmune diseases. The majority of patients with hematologic malignancies diagnosed with PML received rituximab in combination with chemotherapy or as part of a hematopoietic stem cell transplant. The patients with autoimmune diseases had prior or concurrent immunosuppressive therapy. Most cases of PML were diagnosed within 12 months of their last infusion of rituximab.
Consider the diagnosis of PML in any patient presenting with new-onset neurologic manifestations. Evaluation of PML includes, but is not limited to, consultation with a neurologist, brain MRI, and lumbar puncture.
Discontinue RIABNI and consider discontinuation or reduction of any concomitant chemotherapy or immunosuppressive therapy in patients who develop PML.
5.5 Tumor Lysis Syndrome (TLS)
Acute renal failure, hyperkalemia, hypocalcemia, hyperuricemia, or hyperphosphatemia from tumor lysis, sometimes fatal, can occur within 12–24 hours after the first infusion of rituximab products in patients with NHL. A high number of circulating malignant cells (greater than or equal to 25,000/mm3) or high tumor burden, confers a greater risk of TLS.
Administer aggressive intravenous hydration and anti-hyperuricemic therapy in patients at high risk for TLS. Correct electrolyte abnormalities, monitor renal function and fluid balance, and administer supportive care, including dialysis as indicated [see Warnings and Precautions (5.8)].
5.6 Infections
Serious, including fatal, bacterial, fungal, and new or reactivated viral infections can occur during and following the completion of rituximab product-based therapy. Infections have been reported in some patients with prolonged hypogammaglobulinemia (defined as hypogammaglobulinemia greater than 11 months after rituximab exposure). New or reactivated viral infections included cytomegalovirus, herpes simplex virus, parvovirus B19, varicella zoster virus, West Nile virus, and hepatitis B and C. Discontinue RIABNI for serious infections and institute appropriate anti-infective therapy [see Adverse Reactions (6.1, 6.3)]. RIABNI is not recommended for use in patients with severe, active infections.
5.7 Cardiovascular Adverse Reactions
Cardiac adverse reactions, including ventricular fibrillation, myocardial infarction, and cardiogenic shock may occur in patients receiving rituximab products. Discontinue infusions for serious or life-threatening cardiac arrhythmias. Perform cardiac monitoring during and after all infusions of RIABNI for patients who develop clinically significant arrhythmias, or who have a history of arrhythmia or angina [see Adverse Reactions (6.1)].
5.8 Renal Toxicity
Severe, including fatal, renal toxicity can occur after rituximab product administration in patients with NHL. Renal toxicity has occurred in patients who experience tumor lysis syndrome and in patients with NHL administered concomitant cisplatin therapy during clinical trials. The combination of cisplatin and RIABNI is not an approved treatment regimen. Monitor closely for signs of renal failure and discontinue RIABNI in patients with a rising serum creatinine or oliguria [see Warnings and Precautions (5.5)].
5.9 Bowel Obstruction and Perforation
Abdominal pain, bowel obstruction and perforation, in some cases leading to death, can occur in patients receiving rituximab products in combination with chemotherapy. In postmarketing reports, the mean time to documented gastrointestinal perforation was 6 (range 1–77) days in patients with NHL. Evaluate if symptoms of obstruction such as abdominal pain or repeated vomiting occur.
5.10 Immunization
The safety of immunization with live viral vaccines following rituximab product therapy has not been studied and vaccination with live virus vaccines is not recommended before or during treatment.
For patients treated with RIABNI, physicians should review the patient's vaccination status and patients should, if possible, be brought up-to-date with all immunizations in agreement with current immunization guidelines prior to initiating RIABNI and administer non-live vaccines at least 4 weeks prior to a course of RIABNI.
The effect of rituximab on immune responses was assessed in a randomized, controlled study in patients with RA treated with rituximab and methotrexate (MTX) compared to patients treated with MTX alone. A response to pneumococcal vaccination (a T-cell independent antigen) as measured by an increase in antibody titers to at least 6 of 12 serotypes was lower in patients treated with rituximab plus MTX as compared to patients treated with MTX alone (19% vs. 61%). A lower proportion of patients in the rituximab plus MTX group developed detectable levels of anti-keyhole limpet hemocyanin antibodies (a novel protein antigen) after vaccination compared to patients on MTX alone (47% vs. 93%).
A positive response to tetanus toxoid vaccine (a T-cell dependent antigen with existing immunity) was similar in patients treated with rituximab plus MTX compared to patients on MTX alone (39% vs. 42%). The proportion of patients maintaining a positive Candida skin test (to evaluate delayed type hypersensitivity) was also similar (77% of patients on rituximab plus MTX vs. 70% of patients on MTX alone).
Most patients in the rituximab-treated group had B-cell counts below the lower limit of normal at the time of immunization. The clinical implications of these findings are not known.
5.11 Embryo-Fetal Toxicity
Based on human data, rituximab products can cause fetal harm due to B-cell lymphocytopenia in infants exposed in utero. Advise pregnant women of the potential risk to a fetus. Advise females of reproductive potential to use effective contraception while receiving RIABNI and for 12 months after the last dose [see Use in Specific Populations (8.1, 8.3)].
5.12 Concomitant Use with Other Biologic Agents and DMARDS other than Methotrexate in RA, GPA and MPA
Limited data are available on the safety of the use of biologic agents or disease modifying antirheumatic drugs (DMARDs) other than methotrexate in RA patients exhibiting peripheral B-cell depletion following treatment with rituximab. Observe patients closely for signs of infection if biologic agents and/or DMARDs are used concomitantly. Use of concomitant immunosuppressants other than corticosteroids has not been studied in GPA or MPA patients exhibiting peripheral B-cell depletion following treatment with rituximab products.
5.13 Use in RA Patients Who Have Not Had Prior Inadequate Response to Tumor Necrosis Factor (TNF) Antagonists
While the efficacy of rituximab was supported in four controlled trials in patients with RA with prior inadequate responses to non-biologic DMARDs, and in a controlled trial in MTX-naïve patients, a favorable risk-benefit relationship has not been established in these populations. The use of RIABNI in patients with RA who have not had prior inadequate response to one or more TNF antagonists is not recommended [see Clinical Studies (14.6)].
6. Adverse Reactions/Side Effects
The following clinically significant adverse reactions are discussed in greater detail in other sections of the labeling:
- Infusion-related reactions [see Warnings and Precautions (5.1)]
- Severe mucocutaneous reactions [see Warnings and Precautions (5.2)]
- Hepatitis B reactivation with fulminant hepatitis [see Warnings and Precautions (5.3)]
- Progressive multifocal leukoencephalopathy [see Warnings and Precautions (5.4)]
- Tumor lysis syndrome [see Warnings and Precautions (5.5)]
- Infections [see Warnings and Precautions (5.6)]
- Cardiovascular adverse reactions [see Warnings and Precautions (5.7)]
- Renal toxicity [see Warnings and Precautions (5.8)]
- Bowel obstruction and perforation [see Warnings and Precautions (5.9)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
Rheumatoid Arthritis
The data presented below reflect the experience in 2578 RA patients treated with rituximab in controlled and long-term studies1 with a total exposure of 5014 patient-years.
Among all exposed patients, adverse reactions reported in greater than 10% of patients include infusion-related reactions, upper respiratory tract infection, nasopharyngitis, urinary tract infection, and bronchitis.
In placebo-controlled studies, patients received 2 × 500 mg or 2 × 1,000 mg intravenous infusions of rituximab or placebo, in combination with methotrexate, during a 24-week period. From these studies, 938 patients treated with rituximab (2 × 1,000 mg) or placebo have been pooled (see Table 3). Adverse reactions reported in greater than or equal to 5% of patients were hypertension, nausea, upper respiratory tract infection, arthralgia, pyrexia and pruritus (see Table 3). The rates and types of adverse reactions in patients who received rituximab 2 × 500 mg were similar to those observed in patients who received rituximab 2 × 1,000 mg.
| Adverse Reactions | Placebo + MTX N=398 n (%) | Rituximab + MTX N=540 n (%) |
|---|---|---|
|
||
| Hypertension | 21 (5) | 43 (8) |
| Nausea | 19 (5) | 41 (8) |
| Upper Respiratory Tract Infection | 23 (6) | 37 (7) |
| Arthralgia | 14 (4) | 31 (6) |
| Pyrexia | 8 (2) | 27 (5) |
| Pruritus | 5 (1) | 26 (5) |
| Chills | 9 (2) | 16 (3) |
| Dyspepsia | 3 (< 1) | 16 (3) |
| Rhinitis | 6 (2) | 14 (3) |
| Paresthesia | 3 (< 1) | 12 (2) |
| Urticaria | 3 (< 1) | 12 (2) |
| Abdominal Pain Upper | 4 (1) | 11 (2) |
| Throat Irritation | 0 (0) | 11 (2) |
| Anxiety | 5 (1) | 9 (2) |
| Migraine | 2 (< 1) | 9 (2) |
| Asthenia | 1 (< 1) | 9 (2) |
- 1
- Pooled studies: NCT00074438, NCT00422383, NCT00468546, NCT00299130, NCT00282308, NCT00266227, NCT02693210, NCT02093026 and NCT02097745.
Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
6.2 Immunogenicity
As with all therapeutic proteins, there is a potential for immunogenicity. The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies in the studies described below with the incidence of antibodies in other studies or to other rituximab products may be misleading.
Using an ELISA assay, anti-rituximab antibody was detected in 4 of 356 (1.1%) patients with low-grade or follicular NHL receiving single-agent rituximab. Three of the four patients had an objective clinical response.
A total of 273/2578 (11%) patients with RA tested positive for anti-rituximab antibodies at any time after receiving rituximab. Anti-rituximab antibody positivity was not associated with increased rates of infusion-related reactions or other adverse events. Upon further treatment, the proportions of patients with infusion-related reactions were similar between anti-rituximab antibody positive and negative patients, and most reactions were mild to moderate. Four anti-rituximab antibody positive patients had serious infusion-related reactions, and the temporal relationship between anti-rituximab antibody positivity and infusion-related reaction was variable.
A total of 23/99 (23%) rituximab-treated adult patients with GPA and MPA developed anti-rituximab antibodies by 18 months in GPA/MPA Study 1. The clinical relevance of anti-rituximab antibody formation in rituximab-treated adult patients is unclear.
6.3 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of rituximab. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Hematologic: prolonged pancytopenia, marrow hypoplasia, Grade 3-4 prolonged or late-onset neutropenia, hyperviscosity syndrome in Waldenstrom's macroglobulinemia, prolonged hypogammaglobulinemia [see Warnings and Precautions (5.6)].
- Cardiac: fatal cardiac failure.
- Immune/Autoimmune Events: uveitis, optic neuritis, systemic vasculitis, pleuritis, lupus-like syndrome, serum sickness, polyarticular arthritis, and vasculitis with rash.
- Infection: viral infections, including progressive multifocal leukoencephalopathy (PML), increase in fatal infections in HIV-associated lymphoma, and a reported increased incidence of Grade 3 and 4 infections [see Warnings and Precautions (5.6)].
- Neoplasia: disease progression of Kaposi's sarcoma.
- Skin: severe mucocutaneous reactions, pyoderma gangrenosum (including genital presentation).
- Gastrointestinal: bowel obstruction and perforation.
- Pulmonary: fatal bronchiolitis obliterans and fatal interstitial lung disease.
- Nervous system: Posterior Reversible Encephalopathy Syndrome (PRES)/Reversible Posterior Leukoencephalopathy Syndrome (RPLS).
7. Drug Interactions
Formal drug interaction studies have not been performed with rituximab products. In patients with CLL, rituximab did not alter systemic exposure to fludarabine or cyclophosphamide. In clinical trials of patients with RA, concomitant administration of methotrexate or cyclophosphamide did not alter the pharmacokinetics of rituximab.
8. Use In Specific Populations
8.2 Lactation
There are limited data on the presence of rituximab in human milk and the effect on the breastfed child, and there are no data on the effect on milk production. Rituximab is detected in the milk of lactating cynomolgus monkeys, and maternal IgG is present in human breast milk. Rituximab has also been reported to be excreted at low concentrations in human breast milk. Given that the clinical significance of this finding for children is not known, advise women not to breastfeed during treatment with RIABNI and for 6 months after the last dose due to the potential of serious adverse reactions in breastfed children.
8.3 Females and Males of Reproductive Potential
Rituximab products can cause fetal harm when administered to a pregnant woman [see Use in Specific Populations (8.1)].
8.4 Pediatric Use
A pediatric assessment for RIABNI demonstrates that RIABNI is safe and effective for pediatric patients in an indication for which Rituxan (rituximab) is approved. However, RIABNI is not approved for such indication due to marketing exclusivity for Rituxan (rituximab). The safety and effectiveness of rituximab products, including RIABNI, have not been established in pediatric patients less than 2 years of age for GPA and MPA.
The safety and effectiveness of rituximab products, including RIABNI, have not been established in pediatric patients with CLL.
8.5 Geriatric Use
11. Riabni Description
Rituximab-arrx is a genetically engineered chimeric murine/human monoclonal IgG1 kappa antibody directed against the CD20 antigen. Rituximab-arrx has an approximate molecular weight of 145 kD.
Rituximab-arrx is produced in a mammalian cell (Chinese Hamster Ovary) suspension culture in a nutrient medium.
RIABNI (rituximab-arrx) injection is a sterile, preservative-free, clear to slightly opalescent, colorless to slightly yellow solution for intravenous infusion. RIABNI is supplied at a concentration of 10 mg/mL in either 100 mg/10 mL or 500 mg/50 mL single-dose vials. Each mL of solution contains 10 mg rituximab-arrx, polysorbate 80 (0.7 mg), sodium chloride (9 mg), sodium citrate dihydrate (7.35 mg), and Water for Injection, USP. Hydrochloric acid is used to adjust the buffer solution pH. The pH is 6.5.
12. Riabni - Clinical Pharmacology
12.1 Mechanism of Action
Rituximab-arrx is a monoclonal antibody. Rituximab products target the CD20 antigen expressed on the surface of pre-B and mature B-lymphocytes. Upon binding to CD20, rituximab products mediate B-cell lysis. Possible mechanisms of cell lysis include complement dependent cytotoxicity (CDC) and antibody dependent cell mediated cytotoxicity (ADCC). B cells are believed to play a role in the pathogenesis of rheumatoid arthritis (RA) and associated chronic synovitis. In this setting, B cells may be acting at multiple sites in the autoimmune/inflammatory process, including through production of rheumatoid factor (RF) and other autoantibodies, antigen presentation, T-cell activation, and/or proinflammatory cytokine production.
14. Clinical Studies
14.1 Relapsed or Refractory, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
The safety and effectiveness of rituximab in relapsed, refractory CD20+ NHL were demonstrated in 3 single-arm studies enrolling 296 patients.
14.2 Previously Untreated, Low-Grade or Follicular, CD20-Positive, B-Cell NHL
The safety and effectiveness of rituximab in previously untreated, low-grade or follicular, CD20+ NHL were demonstrated in 3 randomized, controlled trials enrolling 1,662 patients.
NHL Study 5
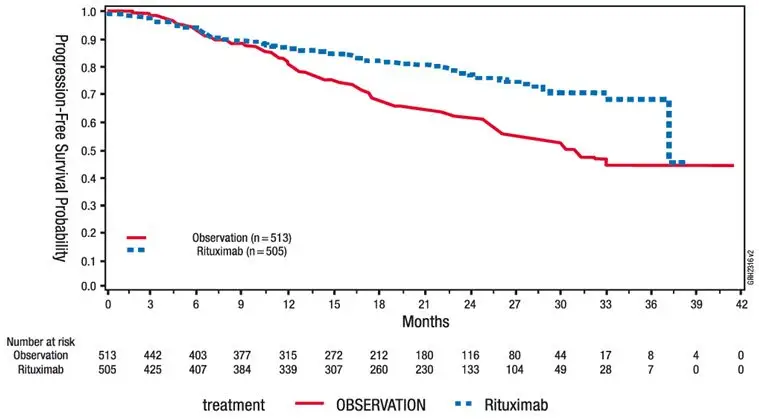
An open-label, multicenter, randomized (1:1) study was conducted in 1,018 patients with previously untreated follicular NHL who achieved a response (CR or PR) to rituximab in combination with chemotherapy. Patients were randomized to rituximab as single-agent maintenance therapy, 375 mg/m2 every 8 weeks for up to 12 doses or to observation. Rituximab was initiated at 8 weeks following completion of chemotherapy. The main outcome measure of the study was progression-free survival (PFS), defined as the time from randomization in the maintenance/observation phase to progression, relapse, or death, as determined by independent review.
Of the randomized patients, 40% were greater than or equal to 60 years of age, 70% had Stage IV disease, 96% had ECOG performance status (PS) 0–1, and 42% had FLIPI scores of 3–5. Prior to randomization to maintenance therapy, patients had received R-CHOP (75%), R-CVP (22%), or R-FCM (3%); 71% had a complete or unconfirmed complete response and 28% had a partial response.
PFS was longer in patients randomized to rituximab as single agent maintenance therapy (HR: 0.54, 95% CI: 0.42, 0.70; see Figure 1). The PFS results based on investigator assessment of progression were similar to those obtained by the independent review assessment.
| Figure 1. Kaplan-Meier Plot of IRC Assessed PFS in NHL Study 5 |
 |
14.3 Diffuse Large B-Cell NHL (DLBCL)
The safety and effectiveness of rituximab were evaluated in three randomized, active-controlled, open-label, multicenter studies with a collective enrollment of 1,854 patients. Patients with previously untreated diffuse large B-cell NHL received rituximab in combination with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or other anthracycline-based chemotherapy regimens.
NHL Study 9
A total of 823 patients with DLBCL, aged 18–60 years, were randomized in a 1:1 ratio to receive an anthracycline-containing chemotherapy regimen alone or in combination with rituximab. The main outcome measure of the study was time to treatment failure, defined as time from randomization to the earliest of progressive disease, failure to achieve a complete response, relapse, or death. Among all enrolled patients, 28% had Stage III–IV disease, 100% had IPI scores of less than or equal to 1, 99% had ECOG performance status of less than 2, 29% had elevated LDH levels, 49% had bulky disease, and 34% had extranodal involvement. Efficacy results are presented in Table 8.
| Study 7 (n = 632) | Study 8 (n = 399) | Study 9 (n = 823) |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| R-CHOP | CHOP | R-CHOP | CHOP | R-Chemo | Chemo | ||||||
|
|||||||||||
| Main outcome | Progression-free survival (years) | Event-free survival (years) | Time to treatment failure (years) |
||||||||
| Median of main outcome measure | 3.1 | 1.6 | 2.9 | 1.1 | NE* | NE* | |||||
| Hazard ratio† | 0.69‡ | 0.60‡ | 0.45‡ | ||||||||
| Overall survival at 2 years§ | 74% | 63% | 69% | 58% | 95% | 86% | |||||
| Hazard ratio† | 0.72‡ | 0.68‡ | 0.40‡ | ||||||||
In NHL Study 8, overall survival estimates at 5 years were 58% vs. 46% for R-CHOP and CHOP, respectively.
14.4 Ninety-Minute Infusions in Previously Untreated Follicular NHL and DLBCL
In NHL Study 10, a total of 363 patients with previously untreated follicular NHL (n = 113) or DLBCL (n = 250) were evaluated in a prospective, open-label, multi-center, single-arm trial for the safety of 90-minute rituximab infusions. Patients with follicular NHL received rituximab 375 mg/m2 plus CVP chemotherapy. Patients with DLBCL received rituximab 375 mg/m2 plus CHOP chemotherapy. Patients with clinically significant cardiovascular disease were excluded from the study. Patients were eligible for a 90-minute infusion at Cycle 2 if they did not experience a Grade 3-4 infusion-related adverse event with Cycle 1 and had a circulating lymphocyte count less than or equal to 5,000/mm3 before Cycle 2. All patients were pre-medicated with acetaminophen and an antihistamine and received the glucocorticoid component of their chemotherapy prior to rituximab infusion. The main outcome measure was the development of Grade 3-4 infusion-related reactions on the day of, or day after, the 90-minute infusion at Cycle 2 [see Adverse Reactions (6.1)].
Eligible patients received their Cycle 2 rituximab infusion over 90 minutes as follows: 20% of the total dose given in the first 30 minutes and the remaining 80% of the total dose given over the next 60 minutes [see Dosage and Administration (2.1)]. Patients who tolerated the 90-minute rituximab infusion at Cycle 2 continued to receive subsequent rituximab infusions at the 90-minute infusion rate for the remainder of the treatment regimen (through Cycle 6 or Cycle 8).
The incidence of Grade 3-4 infusion-related reactions at Cycle 2 was 1.1% (95% CI [0.3%, 2.8%]) among all patients, 3.5% (95% CI [1.0%, 8.8%]) for those patients treated with R-CVP, and 0.0% (95% CI [0.0%, 1.5%]) for those patients treated with R-CHOP. For Cycles 2-8, the incidence of Grade 3-4 infusion-related reactions was 2.8% (95% CI [1.3%, 5.0%]). No acute fatal infusion-related reactions were observed.
14.5 Chronic Lymphocytic Leukemia (CLL)
The safety and effectiveness of rituximab were evaluated in two randomized (1:1) multicenter open-label studies comparing FC alone or in combination with rituximab for up to 6 cycles in patients with previously untreated CLL [CLL Study 1 (n = 817)] or previously treated CLL [CLL Study 2 (n = 552)]. Patients received fludarabine 25 mg/m2/day and cyclophosphamide 250 mg/m2/day on days 1, 2 and 3 of each cycle, with or without rituximab. In both studies, seventy-one percent of CLL patients received 6 cycles and 90% received at least 3 cycles of rituximab-based therapy.
In CLL Study 1, 30% of patients were 65 years or older, 31% were Binet stage C, 45% had B symptoms, more than 99% had ECOG performance status (PS) 0–1, 74% were male, and 100% were White. In CLL Study 2, 44% of patients were 65 years or older, 28% had B symptoms, 82% received a prior alkylating drug, 18% received prior fludarabine, 100% had ECOG PS 0–1, 67% were male and 98% were White.
The main outcome measure in both studies was progression-free survival (PFS), defined as the time from randomization to progression, relapse, or death, as determined by investigators (CLL Study 1) or an independent review committee (CLL Study 2). The investigator assessed results in CLL Study 2 were supportive of those obtained by the independent review committee. Efficacy results are presented in Table 9.
| Study 1*
(Previously untreated) | Study 2*
(Previously treated) |
||||||
|---|---|---|---|---|---|---|---|
| R-FC N = 408 | FC N = 409 | R-FC N = 276 | FC N = 276 |
||||
|
|||||||
| Median PFS (months) | 39.8 | 31.5 | 26.7 | 21.7 | |||
| Hazard ratio (95% CI) | 0.56 (0.43, 0.71) | 0.76 (0.6, 0.96) | |||||
| P value (Log-Rank test) | less than 0.01 | 0.02 | |||||
| Response rate (95% CI) | 86% (82, 89) | 73% (68, 77) | 54% (48, 60) | 45% (37, 51) |
|||
Across both studies, 243 of 676 rituximab-treated patients (36%) were 65 years of age or older and 100 rituximab-treated patients (15%) were 70 years of age or older. The results of exploratory subset analyses in elderly patients are presented in Table 10.
| Study 1 | Study 2 | |||
|---|---|---|---|---|
| Age subgroup | Number of Patients | Hazard Ratio for PFS (95% CI) | Number of Patients | Hazard Ratio for PFS (95% CI) |
|
||||
| Age less than 65 yrs | 572 | 0.52 (0.39, 0.70) | 313 | 0.61 (0.45, 0.84) |
| Age greater than or equal to 65 yrs | 245 | 0.62 (0.39, 0.99) | 233 | 0.99 (0.70, 1.40) |
| Age less than 70 yrs | 736 | 0.51 (0.39, 0.67) | 438 | 0.67 (0.51, 0.87) |
| Age greater than or equal to 70 yrs | 81 | 1.17 (0.51, 2.66) | 108 | 1.22 (0.73, 2.04) |
14.6 Rheumatoid Arthritis (RA)
Reducing the Signs and Symptoms: Initial and Re-Treatment Courses
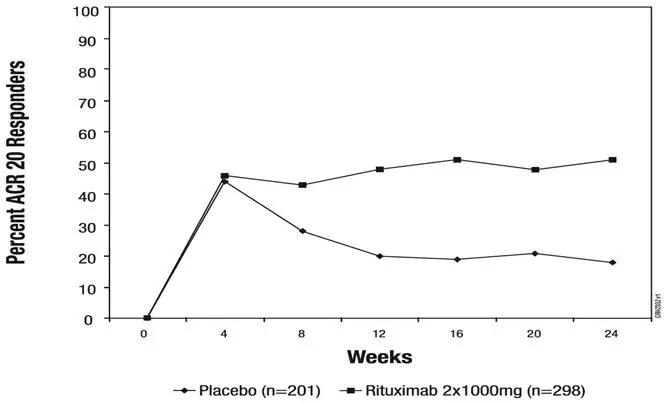
The efficacy and safety of rituximab were evaluated in two randomized, double-blind, placebo-controlled studies of adult patients with moderately to severely active RA who had a prior inadequate response to at least one TNF inhibitor. Patients were 18 years of age or older, diagnosed with active RA according to American College of Rheumatology (ACR) criteria, and had at least 8 swollen and 8 tender joints.
In RA Study 1 (NCT00468546), patients were randomized to receive either rituximab 2×1,000 mg+MTX or placebo +MTX for 24 weeks. Further courses of rituximab 2×1,000 mg+MTX were administered in an open label extension study at a frequency determined by clinical evaluation, but no sooner than 16 weeks after the preceding course of rituximab. In addition to the intravenous premedication, glucocorticoids were administered orally on a tapering schedule from baseline through Day 14. The proportions of patients achieving ACR 20, 50, and 70 responses at Week 24 of the placebo-controlled period are shown in Table 11.
In RA Study 2 (NCT00266227), all patients received the first course of rituximab 2 × 1,000 mg + MTX. Patients who experienced ongoing disease activity were randomized to receive a second course of either rituximab 2 × 1,000 mg + MTX or placebo + MTX, the majority between Weeks 24–28. The proportions of patients achieving ACR 20, 50, and 70 responses at Week 24, before the re-treatment course, and at Week 48, after retreatment, are shown in Table 11.
| Inadequate Response to TNF Antagonists | ||||||||
|---|---|---|---|---|---|---|---|---|
| Study 1 24 Week Placebo-Controlled (Week 24) | Study 2 Placebo-Controlled Retreatment (Week 24 and Week 48) |
|||||||
| Response | Placebo + MTX n = 201 | Rituximab + MTX n = 298 | Treatment Difference (Rituximab – Placebo)*
(95% CI) | Response | Placebo + MTX Retreatment n = 157 | Rituximab + MTX Retreatment n = 318 | Treatment Difference (Rituximab – Placebo)†,‡,*
(95% CI) |
|
|
||||||||
| ACR20 | ACR20 | |||||||
| Week 24 | 18% | 51% | 33% (26%, 41%) | Week 24 | 48% | 45% | NA | |
| Week 48 | 45% | 54% | 11% (2%, 20%) |
|||||
| ACR50 | ACR50 | |||||||
| Week 24 | 5% | 27% | 21% (15%, 27%) | Week 24 | 27% | 21% | NA | |
| Week 48 | 26% | 29% | 4% (-4%, 13%) |
|||||
| ACR70 | ACR70 | |||||||
| Week 24 | 1% | 12% | 11% (7%, 15%) | Week 24 | 11% | 8% | NA | |
| Week 48 | 13% | 14% | 1% (-5%, 8%) |
|||||
Improvement was also noted for all components of ACR response following treatment with rituximab, as shown in Table 12.
| Inadequate Response to TNF Antagonists | ||||
|---|---|---|---|---|
| Parameter (median) | Placebo + MTX (n = 201) | Rituximab + MTX (n = 298) |
||
| Baseline | Wk 24 | Baseline | Wk 24 | |
|
||||
| Tender Joint Count | 31.0 | 27.0 | 33.0 | 13.0 |
| Swollen Joint Count | 20.0 | 19.0 | 21.0 | 9.5 |
| Physician Global Assessment* | 71.0 | 69.0 | 71.0 | 36.0 |
| Patient Global Assessment* | 73.0 | 68.0 | 71.0 | 41.0 |
| Pain* | 68.0 | 68.0 | 67.0 | 38.5 |
| Disability Index (HAQ)† | 2.0 | 1.9 | 1.9 | 1.5 |
| CRP (mg/dL) | 2.4 | 2.5 | 2.6 | 0.9 |
The time course of ACR 20 response for RA Study 1 is shown in Figure 2. Although both treatment groups received a brief course of intravenous and oral glucocorticoids, resulting in similar benefits at Week 4, higher ACR 20 responses were observed for the rituximab group by Week 8. A similar proportion of patients achieved these responses through Week 24 after a single course of treatment (2 infusions) with rituximab. Similar patterns were demonstrated for ACR 50 and 70 responses.
|
| Figure 2. Percent of Patients Achieving ACR 20 Response by Visit* RA Study 1 (Inadequate Response to TNF Antagonists) |
 |
14.7 Granulomatosis with Polyangiitis (GPA) (Wegener's Granulomatosis) and Microscopic Polyangiitis (MPA)
16. How is Riabni supplied
RIABNI (rituximab-arrx) injection is a sterile, clear to slightly opalescent, colorless to slightly yellow preservative free solution for intravenous use supplied as a carton containing one 100 mg/10 mL (10 mg/mL) single dose vial (NDC 55513-224-01) and a carton containing one 500 mg/50 mL (10 mg/mL) single dose vial (NDC 55513-326-01).
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
| This Medication Guide has been approved by the U.S. Food and Drug Administration. | Revised: 6/2022 | ||
| MEDICATION GUIDE
RIABNI™(re AB nee) (rituximab-arrx) injection |
|||
| What is the most important information I should know about RIABNI?
RIABNI can cause serious side effects that can lead to death, including:
|
|||
|
|
||
|
|||
|
|
||
| See "What are the possible side effects of RIABNI?" for more information about side effects. | |||
| What is RIABNI?
RIABNI is a prescription medicine used to treat:
|
|||
Before you receive RIABNI, tell your healthcare provider about all of your medical conditions, including if you:
|
|||
|
|
||
|
|||
How will I receive RIABNI?
|
|||
| What are the possible side effects of RIABNI? RIABNI can cause serious side effects, including:
|
|||
|
|
||
The most common side effects of RIABNI include:
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088. |
|||
| General information about the safe and effective use of RIABNI.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about RIABNI that is written for healthcare providers. |
|||
| What are the ingredients in RIABNI?
Active ingredient: rituximab-arrx Inactive ingredients: polysorbate 80, sodium chloride, sodium citrate dihydrate, and Water for Injection, USP. Hydrochloric acid is used to adjust the buffer solution pH. Manufactured by: Amgen, Inc., One Amgen Center Drive, Thousand Oaks, CA 91320-1799 U.S. License Number 1080 RIABNI™ is a registered trademark of Amgen, Inc. © 2020, 2022 Amgen, Inc. For more information, go to www.RIABNI.com or call 1-805-447-1000. 1XXXXXX – V2 |
|||
| RIABNI
rituximab-arrx injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| RIABNI
rituximab-arrx injection, solution |
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
|
||||||||||||||||||||
| Labeler - Amgen Inc (039976196) |