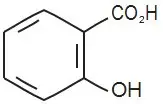
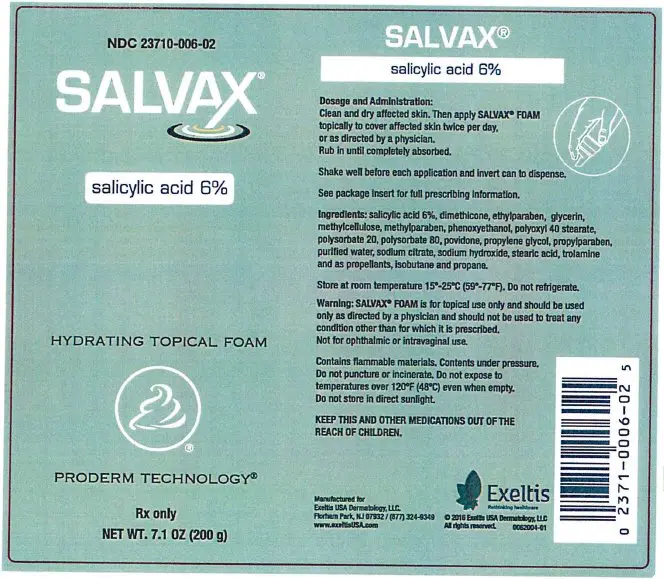
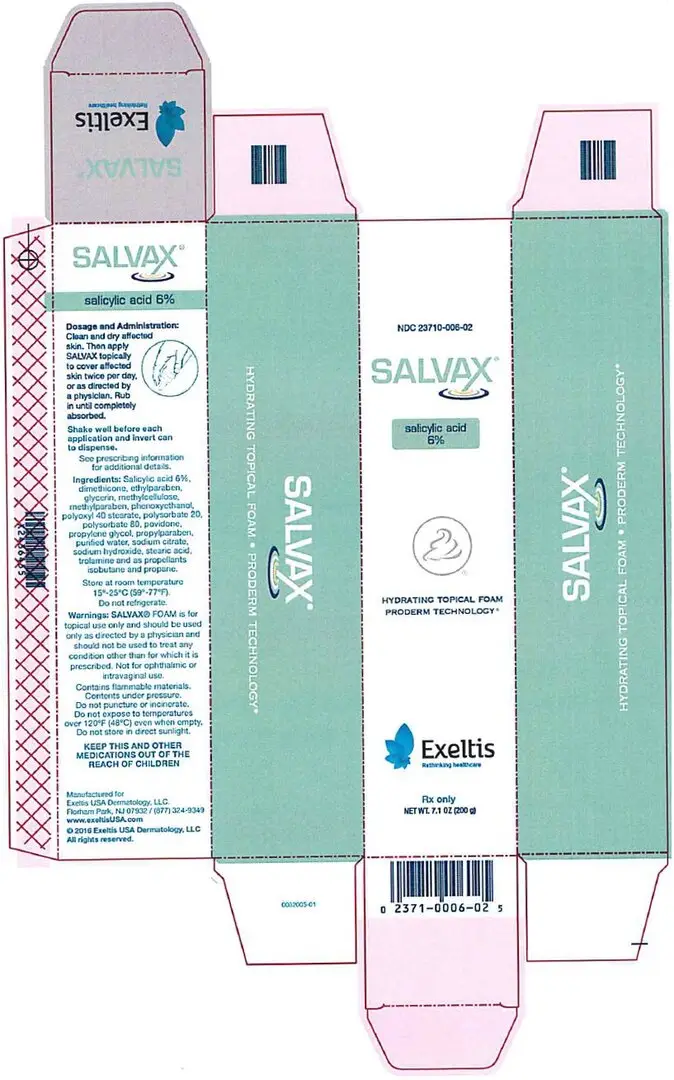
Warnings
SALVAX is for external use only. It is not for ophthalmic, oral, anal
or intravaginal use. Contact with eyes, lips, broken or inflamed skin, and all mucous membranes should be avoided. SALVAX should not be used by persons who have a known hypersensitivity to salicylic acid or any of the other listed ingredients.
Prolonged use over large areas, especially in children and those
patients with significant renal or hepatic impairment could result in salicylism. Concomitant use of other drugs which may contribute to elevated serum salicylate levels should be avoided where the potential for toxicity is present. In children under 12 years of age and those patients with renal or hepatic impairment, the area to be treated should be limited and the patient monitored closely for signs of salicylate toxicity: nausea, vomiting, dizziness, loss of hearing, tinnitus, lethargy, hyperpnoea, diarrhea, psychic disturbances. In the event of salicylic acid toxicity, the use of SALVAX should be discontinued. Fluids should be administered to promote urinary excretion. Treatment with sodium bicarbonate (oral or intravenous) should be instituted as appropriate.
Considering the potential risk of developing Reye's syndrome, salicylate products should not be administered to children or teenagers with varicella or influenza, unless directed by a physician.