Drug Detail:Sancuso (Granisetron (transdermal) [ gra-nis-e-tron ])
Drug Class: 5HT3 receptor antagonists
Highlights of Prescribing Information
SANCUSO (granisetron transdermal system)
Initial U.S. Approval: 2008
Indications and Usage for Sancuso
Sancuso is a serotonin-3 (5-HT3) receptor antagonist indicated for the prevention of nausea and vomiting in adults receiving moderately and/or highly emetogenic chemotherapy for up to 5 consecutive days. (1)
Sancuso Dosage and Administration
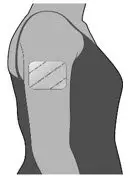
The recommended dosage is a single transdermal system applied to the upper outer arm a minimum of 24 hours, up to a maximum of 48 hours, before chemotherapy. The transdermal system should be worn at minimum, 24 hours after chemotherapy is finished. The transdermal system can be worn for up to 7 days. (2)
Dosage Forms and Strengths
Transdermal System: 3.1 mg per 24 hours. (3)
Contraindications
Known hypersensitivity to granisetron or to any of the components of the transdermal system (4)
Warnings and Precautions
- Granisetron may mask a progressive ileus and/or gastric distention; consider before use in patients with abdominal surgery. Monitor for decreased bowel activity, particularly in patients with risk factors for gastrointestinal obstruction. (5.1)
- Serotonin syndrome has been reported with 5-HT3 receptor antagonists alone but particularly with concomitant use of serotonergic drugs. If such symptoms occur, discontinue Sancuso and initiate supportive treatment. If concomitant use of Sancuso with other serotonergic drugs is clinically warranted, patients should be aware of a potential increased risk of serotonin syndrome. (5.2, 7.1)
- Mild application site reactions have occurred; remove Sancuso transdermal system if severe reactions or a generalized skin reaction occur. (5.3)
- Avoid exposing Sancuso transdermal system and surrounding area to direct external heat sources, such as heating pads (5.4).
- Avoid direct exposure of application site to natural or artificial sunlight, including sunlamps, by covering with clothing throughout the period of wear and for 10 days after removal. (5.5)
Adverse Reactions/Side Effects
The most common adverse reaction (≥ 3%) is constipation. (6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Kyowa Kirin, Inc. at 1-800-SANCUSO (1-800-726-2876) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
See 17 for PATIENT COUNSELING INFORMATION and FDA-approved patient labeling.
Revised: 12/2022
Related/similar drugs
ondansetron, lorazepam, dexamethasone, Zofran, Ativan, metoclopramideFull Prescribing Information
1. Indications and Usage for Sancuso
Sancuso® is indicated for the prevention of nausea and vomiting in adults receiving moderately and/or highly emetogenic chemotherapy regimens of up to 5 consecutive days duration.
2. Sancuso Dosage and Administration
The recommended dosage is a single transdermal system applied to the upper outer arm a minimum of 24 hours, up to a maximum of 48 hours, before chemotherapy. The transdermal system should be worn at minimum, 24 hours after chemotherapy is finished. The transdermal system can be worn for up to 7 days.
3. Dosage Forms and Strengths
Transdermal System: a 52 cm2 thin, translucent, rectangular-shaped transdermal system with rounded corners imprinted on one side with "Granisetron 3.1 mg/24 hours". The transdermal system releases 3.1 mg of granisetron per 24 hours for up to 7 days.
4. Contraindications
Sancuso is contraindicated in patients with known hypersensitivity to granisetron or to any of the components of the transdermal system [see Description (10)].
5. Warnings and Precautions
5.1 Progressive Ileus and Gastric Distention
Sancuso may mask a progressive ileus and/or gastric distention. This should be particularly considered before use of Sancuso in patients who have had recent abdominal surgery. Monitor for decreased bowel activity, particularly in patients with risk factors for gastrointestinal obstruction.
5.2 Serotonin Syndrome
The development of serotonin syndrome has been reported with 5-HT3 receptor antagonists. Most reports have been associated with concomitant use of serotonergic drugs (e.g., selective serotonin reuptake inhibitors (SSRIs), serotonin and norepinephrine reuptake inhibitors (SNRIs), monoamine oxidase inhibitors, mirtazapine, fentanyl, lithium, tramadol, and intravenous methylene blue). Some of the reported cases were fatal. Serotonin syndrome occurring with overdose of another 5-HT3 receptor antagonist alone has also been reported. The majority of reports of serotonin syndrome related to 5-HT3 receptor antagonist use occurred in a post- anesthesia care unit or an infusion center.
Symptoms associated with serotonin syndrome may include the following combination of signs and symptoms: mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, with or without gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Patients should be monitored for the emergence of serotonin syndrome, especially with concomitant use of Sancuso and other serotonergic drugs. If symptoms of serotonin syndrome occur, discontinue Sancuso and initiate supportive treatment. Patients should be informed of the increased risk of serotonin syndrome, especially if Sancuso is used concomitantly with other serotonergic drugs. [see Drug Interactions (7)].
5.3 Skin Reactions
In clinical trials with Sancuso, application site reactions were reported that were generally mild in intensity and did not lead to discontinuation of use. The incidence of reactions was comparable with placebo.
If severe reactions, or a generalized skin reaction occur (e.g., allergic rash, including erythematous, macular, papular rash or pruritus), remove the Sancuso transdermal system.
5.4 Increased Drug Exposure with Use of External Heat Sources
Prolonged exposure to heat results in increasing plasma concentrations of granisetron during the period of heat exposure [see Clinical Pharmacology (12.3)]. Do not apply a heat pad or heat lamp over or in the vicinity of the Sancuso transdermal system and avoid extended exposure to heat [see Dosage and Administration (2)].
5.5 Phototoxicity with Ultraviolet Light Exposure
Granisetron may be affected by direct natural or artificial sunlight, including sunlamps. An in vitro study using Chinese hamster ovary cells suggests that granisetron has the potential for photogenotoxicity [see Nonclinical Toxicology (13.3)]. To avoid a potential skin reaction, advise patients to cover the application site of the transdermal system with clothing if there is a risk of exposure to direct natural or artificial sunlight throughout the period of wear and for 10 days following its removal.
6. Adverse Reactions/Side Effects
The following are serious or otherwise clinically significant adverse reactions reported in other sections of labeling:
- Progressive ileus and gastric distention [see Warnings and Precautions (5.1)]
- Serotonin syndrome [see Warnings and Precautions (5.2)]
- Skin reactions [see Warnings and Precautions (5.3)]
- Increased drug exposure with use of external heat sources [see Warnings and Precautions (5.4)]
- Phototoxicity with ultraviolet light exposure [se Warnings and Precautions (5.5)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of Sancuso was evaluated in a total of 404 patients undergoing chemotherapy who participated in two double-blind, comparator studies with transdermal system treatment durations of up to 7 days. The control groups included a total of 406 patients who received a daily dose of 2 mg oral granisetron, for 1 to 5 days.
Adverse reactions occurred in 9% (35/404) of patients receiving Sancuso and 7% (29/406) of patients receiving oral granisetron. The most common adverse reaction was constipation that occurred in 5% of patients in the Sancuso group and 3% of patients in the oral granisetron group.
Table 1 lists the adverse reactions that occurred in at least 3% of patients treated with Sancuso or oral granisetron.
| Body System
Preferred Term | Sancuso Transdermal System N=404 (%) | Oral granisetron N=406 (%) |
|---|---|---|
| Gastrointestinal disorders | ||
| Constipation | 5 | 3 |
| Nervous system disorders | ||
| Headache | 1 | 3 |
5-HT3 receptor antagonists, such as granisetron, may be associated with arrhythmias or ECG abnormalities. Three ECGs were performed on 588 patients in a randomized, parallel group, double-blind, double-dummy study: at baseline before treatment, the first day of chemotherapy, and 5 to 7 days after starting chemotherapy. QTcF prolongation greater than 450 milliseconds was seen in a total of 11 (1.9%) patients after receiving granisetron, 8 (2.7%) on oral granisetron, and 3 (1.1%) on the transdermal system. No new QTcF prolongation greater than 480 milliseconds was observed in any patient in this study. No arrhythmias were detected in this study.
Adverse reactions reported in clinical trials with other formulations of granisetron include the following:
Gastrointestinal: abdominal pain, diarrhea, constipation, elevation of ALT and AST levels, nausea and vomiting
Cardiovascular: hypertension, hypotension, angina pectoris, atrial fibrillation and syncope have been observed rarely
Central Nervous System: dizziness, insomnia, headache, anxiety, somnolence and asthenia
Hypersensitivity: rare cases of hypersensitivity reactions, sometimes severe (e.g. anaphylaxis, shortness of breath, hypotension, urticaria) have been reported
Other: fever; events often associated with chemotherapy have also been reported: leucopenia, decreased appetite, anemia, alopecia, thrombocytopenia.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of Sancuso. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
General Disorders and Administration Site Conditions: Application site reactions (pain, pruritus, erythema, rash, irritation, vesicles, burn, discoloration, urticaria) [see Warnings and Precautions (5.3)]; transdermal system non-adhesion.
Cardiac Disorders: bradycardia, chest pain, palpitations, sick sinus syndrome
7. Drug Interactions
7.1 Serotonergic Drugs
Serotonin syndrome (including altered mental status, autonomic instability, and neuromuscular symptoms) has been described following the concomitant use of 5-HT3 receptor antagonists and other serotonergic drugs, including selective serotonin reuptake inhibitors (SSRIs) and serotonin and noradrenaline reuptake inhibitors (SNRIs). Monitor for the emergence of serotonin syndrome. If symptoms occur, discontinue Sancuso and initiate supportive treatment [see Warnings and Precautions (5.4)].
7.2 Concomitant Use Medications
There have been no definitive drug-drug interaction studies to examine pharmacokinetic or pharmacodynamic interaction with other drugs. However, in humans, granisetron hydrochloride injection has been safely administered with drugs representing benzodiazepines, neuroleptics and anti-ulcer medications commonly prescribed with antiemetic treatments. Granisetron hydrochloride injection also does not appear to interact with emetogenic cancer therapies. In agreement with these data, no clinically relevant drug interactions have been reported in clinical studies with Sancuso.
8. Use In Specific Populations
8.4 Pediatric Use
Safety and effectiveness of Sancuso have not been established in pediatric patients.
8.5 Geriatric Use
Clinical studies of Sancuso did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, cautious treatment selection for an elderly patient is prudent because of the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
8.6 Renal Impairment or Hepatic Impairment
Although no studies have been performed to investigate the pharmacokinetics of Sancuso in patients with renal or hepatic impairment, pharmacokinetic information is available for intravenous granisetron [see Clinical Pharmacology (12.3)]. No dosage adjustment is recommended for renal or hepatic impairment.
10. Overdosage
There is no specific antidote for granisetron overdosage. In the case of overdosage, symptomatic treatment should be given.
11. Sancuso Description
Sancuso contains granisetron, which is a serotonin-3 (5-HT3) receptor antagonist. Chemically it is 1-methyl-N-[(1R,3r,5S)-9-methyl-9-azabicyclo[3.3.1]non-3-yl]-1H-indazole-3-carboxamide with a molecular weight of 312.4. Its empirical formula is C18H24N4O, while its chemical structure is:
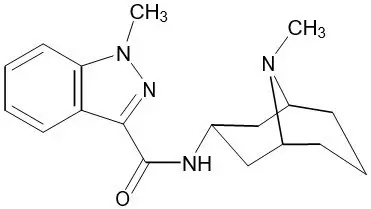 |
| Granisetron |
Granisetron is a white to off-white solid that is insoluble in water. The inactive ingredients are acrylate-vinylacetate copolymer, polyester, titanium dioxide, polyamide resin and polyethylene wax. Sancuso is a 52 cm2 thin, translucent, matrix-type transdermal system that is rectangular- shaped with rounded corners, consisting of a backing (polyester), the drug matrix (acrylate- vinylacetate copolymer) and a release liner (siliconized polyester).
12. Sancuso - Clinical Pharmacology
12.1 Mechanism of Action
Granisetron is a selective 5-hydroxytryptamine3 (5-HT3) receptor antagonist with little or no affinity for other serotonin receptors, including 5-HT1, 5-HT1A, 5-HT1B/C, 5-HT2; for alpha1-, alpha2-, or beta-adrenoreceptors; for dopamine-D2; or for histamine-H1; benzodiazepine; picrotoxin or opioid receptors.
Serotonin receptors of the 5-HT3 type are located peripherally on vagal nerve terminals and centrally in the chemoreceptor trigger zone of the area postrema. During chemotherapy that induces vomiting, mucosal enterochromaffin cells release serotonin, which stimulates 5-HT3 receptors. This evokes vagal afferent discharge, inducing vomiting. Animal studies demonstrate that, in binding to 5-HT3 receptors, granisetron blocks serotonin stimulation and subsequent vomiting after emetogenic stimuli such as cisplatin. In the ferret animal model, a single granisetron injection prevented vomiting due to high-dose cisplatin or arrested vomiting within 5 to 30 seconds.
12.2 Pharmacodynamics
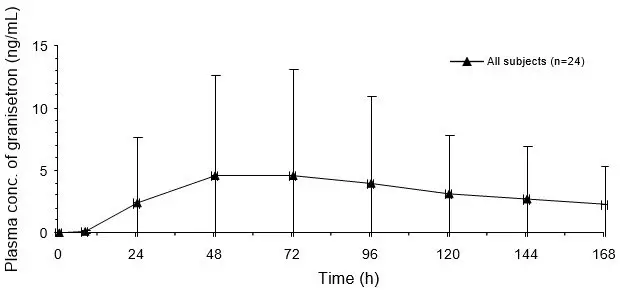
The effect of granisetron on QTc prolongation was evaluated in a randomized, single-blind, positive (moxifloxacin 400 mg) - and placebo controlled parallel study in healthy subjects. A total of 120 subjects were administered Sancuso transdermal system (n=60) or intravenous granisetron (10 mcg/kg over 30 seconds; n=60). In a study with demonstrated ability to detect small effects, the upper bound of the 90% confidence interval for the largest placebo adjusted, baseline corrected QTc based on Fridericia correction method (QTcF) for Sancuso was below 10 ms. This study suggests that Sancuso does not have significant effects on QT prolongation.
No evidence of an effect on plasma prolactin or aldosterone concentrations has been found in studies using granisetron.
The effect on oro-cecal transit time following application of Sancuso has not been studied. Granisetron hydrochloride injection exhibited no effect on oro-cecal transit time in healthy subjects given a single intravenous infusion of 50 mcg/kg or 200 mcg/kg. Single and multiple oral doses of granisetron hydrochloride slowed colonic transit in healthy subjects.
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
In a 24-month carcinogenicity study, rats were treated orally with granisetron 1, 5 or 50 mg/kg/day (6, 30 or 300 mg/m2/day). The 50 mg/kg/day dose was reduced to 25 mg/kg/day (150 mg/m2/day) during week 59 due to toxicity. For a 50 kg person of average height (1.46 m2 body surface area), these doses represent about 2.6, 13, and 65 times the recommended clinical dose (3.1 mg/day, 2.3 mg/m2/day, delivered by the Sancuso transdermal system, on a body surface area basis). There was a statistically significant increase in the incidence of hepatocellular carcinomas and adenomas in males treated with 5 mg/kg/day (30 mg/m2/day, about 13 times the recommended human dose with Sancuso, on a body surface area basis) and above, and in females treated with 25 mg/kg/day (150 mg/m2/day, about 65 times the recommended human dose with Sancuso, on a body surface area basis). No increase in liver tumors was observed at a dose of 1 mg/kg/day (6 mg/m2/day, about 2.6 times the recommended human dose with Sancuso, on a body surface area basis) in males and 5 mg/kg/day (30 mg/m2/day, about 13 times the recommended human dose with Sancuso, on a body surface area basis) in females.
In a 12-month oral toxicity study, treatment with granisetron 100 mg/kg/day (600 mg/m2/day, about 261 times the recommended human dose with Sancuso, on a body surface area basis) produced hepatocellular adenomas in male and female rats while no such tumors were found in the control rats. A 24-month mouse carcinogenicity study of granisetron did not show a statistically significant increase in tumor incidence, but the study was not conclusive.
Because of the tumor findings in rat studies, Sancuso should be prescribed only at the dose and for the indication recommended [see Indications and Usage (1), Dosage and Administration (2)].
Granisetron was not mutagenic in an in vitro Ames test and mouse lymphoma cell forward mutation assay, and in vivo mouse micronucleus test and in vitro and ex vivo rat hepatocyte UDS assays. It, however, produced a significant increase in UDS in HeLa cells in vitro and a significant increased incidence of cells with polyploidy in an in vitro human lymphocyte chromosomal aberration test.
Granisetron at subcutaneous doses up to 6 mg/kg/day (36 mg/m2/day, about 16 times the recommended human dose of Sancuso, on a body surface area basis), and oral doses up to 100 mg/kg/day (600 mg/m2/day, about 261 times the recommended human dose of Sancuso, on a body surface area basis) was found to have no effect on fertility and reproductive performance of male and female rats.
13.3 Phototoxicity
When tested for potential photogenotoxicity in vitro in a Chinese hamster ovary (CHO) cell line, at 200 and 300 mcg/mL, granisetron increased the percentage of cells with chromosomal aberration following photoirradiation [see Warnings and Precautions (5.5)].
Granisetron was not phototoxic when tested in vitro in a mouse fibroblast cell line. When tested in vivo in guinea-pigs, Sancuso transdermal system did not show any potential for photoirritation or photosensitivity. No phototoxicity studies have been performed in humans.
14. Clinical Studies
The effectiveness of Sancuso in the prevention of chemotherapy-induced nausea and vomiting (CINV) was evaluated in a randomized, parallel group, double-blind, double-dummy study conducted in the U.S. and abroad. The study compared the efficacy, tolerability and safety of Sancuso transdermal system with that of 2 mg oral granisetron once daily in the prevention of nausea and vomiting in a total of 641 patients receiving multi-day chemotherapy.
The population randomized into the trial included 48% males and 52% females aged 16 to 86 years receiving moderately emetogenic (ME) or highly emetogenic (HE) multi-day chemotherapy. Seventy-eight (78%) of patients were White, 12% Asian, 10% Hispanic/Latino and 0% Black.
Sancuso was applied 24 to 48 hours before the first dose of chemotherapy and kept in place for 7 days. Oral granisetron was administered daily for the duration of the chemotherapy regimen, 1 hour before each dose of chemotherapy. Efficacy was assessed from the first administration until 24 hours after the start of the last day's administration of the chemotherapy regimen.
The primary endpoint of the trial was the proportion of patients achieving no vomiting and/or retching, no more than mild nausea and no rescue medication from the first administration until 24 hours after the start of the last day's administration of multi-day chemotherapy. Using this definition, the effect of Sancuso was established in 60.2% of patients in the Sancuso arm and 64.8% of patients receiving oral granisetron (difference -4.89%; 95% confidence interval – 12.91% to +3.13%).
An assessment of transdermal system adhesion in 621 patients receiving either active or placebo transdermal system showed that less than 1% of transdermal systems became detached over the course of the 7 day period of transdermal system application.
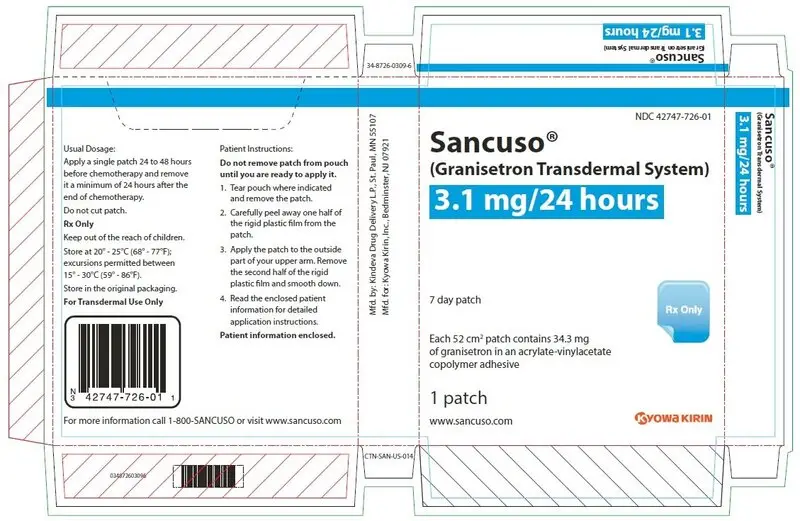
16. How is Sancuso supplied
Sancuso (granisetron transdermal system) is a 52 cm2 thin, translucent, rectangular-shaped transdermal system with rounded corners imprinted on one side with "Granisetron 3.1 mg/24 hours". The transdermal system releases 3.1 mg of granisetron per 24 hours for up to 7 days.
Each Sancuso transdermal system is packaged in a separate sealed foil-lined plastic pouch supplied in packages of 1 (NDC 42747-726-01) transdermal system.
| SANCUSO
granisetron patch |
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||
| Labeler - Kyowa Kirin, Inc. (014778321) |
| Establishment | |||
| Name | Address | ID/FEI | Business Operations |
|---|---|---|---|
| Kindeva Drug Delivery L.P. | 128688199 | MANUFACTURE(42747-726) , PACK(42747-726) , ANALYSIS(42747-726) | |