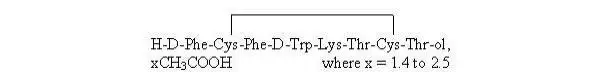General
Sandostatin® (octreotide acetate) Injection alters the balance between the counter-regulatory hormones, insulin, glucagon and growth hormone (GH), which may result in hypoglycemia or hyperglycemia. Sandostatin also suppresses secretion of thyroid stimulating hormone, which may result in hypothyroidism. Cardiac conduction abnormalities have also occurred during treatment with Sandostatin. However, the incidence of these adverse events during long-term therapy was determined vigorously only in acromegaly patients who, due to their underlying disease and/or the subsequent treatment they receive, are at an increased risk for the development of diabetes mellitus, hypothyroidism, and cardiovascular disease. Although the degree to which these abnormalities are related to Sandostatin therapy is not clear, new abnormalities of glycemic control, thyroid function, and electrocardiogram (ECG) developed during Sandostatin therapy, as described below.
Risk of Pregnancy with Normalization of Insulin Growth Factor-1 (IGF-1; somatomedin C) and Growth Hormone (GH)
Although acromegaly may lead to infertility, there are reports of pregnancy in acromegalic women. In women with active acromegaly who have been unable to become pregnant, normalization of GH and IGF-1 (somatomedin C) may restore fertility. Female patients of child-bearing potential should be advised to use adequate contraception during treatment with octreotide.
Hyperglycemia and Hypoglycemia
The hypoglycemia or hyperglycemia which occurs during Sandostatin therapy is usually mild, but may result in overt diabetes mellitus or necessitate dose changes in insulin or other hypoglycemic agents. Hypoglycemia and hyperglycemia occurred on Sandostatin in 3% and 16% of acromegalic patients, respectively. Severe hyperglycemia, subsequent pneumonia, and death following initiation of Sandostatin therapy was reported in one patient with no history of hyperglycemia.
In patients with concomitant Type I diabetes mellitus, Sandostatin Injection may affect glucose regulation, and insulin requirements may be reduced. Symptomatic hypoglycemia, which may be severe, has been reported in these patients. In nondiabetics and Type II diabetics with partially intact insulin reserves, Sandostatin Injection administration may result in decreases in plasma insulin levels and hyperglycemia. It is therefore recommended that glucose tolerance and anti-diabetic treatment be periodically monitored during therapy with these drugs.
Thyroid Function Abnormalities
In acromegalic patients, 12% developed biochemical hypothyroidism only, 8% developed goiter, and 4% required initiation of thyroid replacement therapy while receiving Sandostatin. Baseline and periodic assessment of thyroid function (TSH, total, and/or free T4) is recommended during chronic therapy.
Cardiac Function Abnormalities [see WARNINGS; Complete Atrioventricular Block]
In acromegalics, bradycardia (< 50 bpm) developed in 25%; conduction abnormalities occurred in 10% and arrhythmias occurred in 9% of patients during Sandostatin therapy.
Other electrocardiogram (ECG) changes observed included QT prolongation, axis shifts, early repolarization, low voltage, R/S transition, and early R-wave progression. These ECG changes are not uncommon in acromegalic patients.
Dose adjustments in drugs such as beta-blockers that have bradycardia effects may be necessary.
In one acromegalic patient with severe congestive heart failure, initiation of Sandostatin therapy resulted in worsening of congestive heart failure with improvement when drug was discontinued. Confirmation of a drug effect was obtained with a positive rechallenge.
Pancreatitis
Several cases of pancreatitis have been reported in patients receiving Sandostatin therapy.
Dietary Fats Malabsorption
Sandostatin may alter absorption of dietary fats in some patients.
Decreased Vitamin B12 Levels and Abnormal Schilling’s Tests
Depressed vitamin B12 levels and abnormal Schilling’s tests have been observed in some patients receiving Sandostatin therapy, and monitoring of vitamin B12 levels is recommended during chronic Sandostatin therapy.
Increased Half-life of Sandostatin in Patients with Renal Failure on Dialysis
In patients with severe renal failure requiring dialysis, the half-life of Sandostatin may be increased, necessitating adjustment of the maintenance dosage.