Drug Detail:Secuado (transdermal) (Asenapine (transdermal) [ a-sen-a-peen ])
Drug Class: Atypical antipsychotics
Highlights of Prescribing Information
SECUADO® (asenapine) transdermal system
Initial U.S. Approval: 2009
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
See full prescribing information for complete boxed warning.
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. SECUADO is not approved for the treatment of patients with dementia-related psychosis. (5.1)
Indications and Usage for Secuado Film
SECUADO is an atypical antipsychotic indicated for the treatment of adults with schizophrenia. (1)
Secuado Film Dosage and Administration
- For transdermal use only. (2.2)
- Apply one SECUADO transdermal system every 24 hours. (2.2)
- Apply SECUADO to one of the following sites: the hip, abdomen, upper arm, or upper back area. (2.2)
- The recommended starting dose of SECUADO is 3.8 mg/24 hours. May increase dosage to 5.7 mg/24 hours or 7.6 mg/24 hours after one week. (2.1)
Dosage Forms and Strengths
- Transdermal System: 3.8 mg/24 hours, 5.7 mg/24 hours and 7.6 mg/24 hours (3)
Contraindications
- Severe hepatic impairment (Child-Pugh C). (8.7, 12.3)
- Known hypersensitivity to SECUADO or to any components in the transdermal system. (4, 5.6, 17)
Warnings and Precautions
- Cerebrovascular Adverse Reactions in Elderly Patients with Dementia-Related Psychosis: Increased incidence of cerebrovascular adverse reactions (e.g., stroke, transient ischemic attack). (5.2)
- Neuroleptic Malignant Syndrome: Manage with immediate discontinuation and close monitoring. (5.3)
- Tardive Dyskinesia: Discontinue if clinically appropriate. (5.4)
- Metabolic Changes: Monitor for hyperglycemia/diabetes mellitus, dyslipidemia, and weight gain. (5.5)
- Orthostatic Hypotension: Monitor heart rate and blood pressure and warn patients with known cardiovascular or cerebrovascular disease, and risk of dehydration or syncope. (5.7)
- Leukopenia, Neutropenia, and Agranulocytosis: Perform complete blood counts (CBC) in patients with pre-existing low white blood cell count (WBC) or history of leukopenia or neutropenia. Consider discontinuing SECUADO if a clinically significant decline in WBC occurs in absence of other causative factors. (5.9)
- QT Prolongation: Increases in QT interval; avoid use with drugs that also increase the QT interval and in patients with risk factors for prolonged QT interval. (5.10)
- Seizures: Use cautiously in patients with a history of seizures or with conditions that lower the seizure threshold. (5.12)
- Potential for Cognitive and Motor Impairment: Use caution when operating machinery. (5.13)
- External Heat: Avoid exposing SECUADO to external heat sources during wear because both the rate and extent of absorption are increased. (5.16)
- Application Site Reactions: During wear time or immediately after removal of SECUADO, local skin reactions may occur. Instruct patients to select a different transdermal system application site each day to limit the occurrence of skin reactions. (5.17)
Adverse Reactions/Side Effects
Commonly observed adverse reactions (incidence ≥5% and at least twice that for placebo): Extrapyramidal disorder, application site reaction, and weight gain.(6.1)
To report SUSPECTED ADVERSE REACTIONS, contact Noven Therapeutics, LLC at 1-800-455-8070 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Drug Interactions
- Antihypertensive drugs: SECUADO may enhance antihypertensive effects. Monitor blood pressure and adjust dosage of antihypertensive drug accordingly. (7.1)
- Strong CYP1A2 Inhibitors: Consider dose reduction for SECUADO based on clinical response. (7.1)
- Paroxetine (CYP2D6 substrate and inhibitor): Reduce paroxetine dose by half. (7.1)
Use In Specific Populations
Pregnancy: May cause extrapyramidal and/or withdrawal symptoms in neonates with third trimester exposure. (8.1)
See 17 for PATIENT COUNSELING INFORMATION.
Revised: 11/2022
Full Prescribing Information
WARNING: INCREASED MORTALITY IN ELDERLY PATIENTS WITH DEMENTIA-RELATED PSYCHOSIS
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. SECUADO is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
1. Indications and Usage for Secuado Film
SECUADO is indicated for the treatment of adults with schizophrenia [see Clinical Studies (14)].
2. Secuado Film Dosage and Administration
2.1 Schizophrenia
Initiate SECUADO at a dosage of 3.8 mg/24 hours. In a short-term, placebo-controlled trial, there was no suggestion of added benefit at a dosage of 7.6 mg/24 hours, on average, but there was an increase in certain adverse reactions. The dosage may be increased to 5.7 mg/24 hours or 7.6 mg/24 hours, as needed, after one week. The safety of doses above 7.6 mg/24 hours has not been evaluated in clinical studies [see Clinical Studies (14)].
Based on the average exposure (AUC) of asenapine, SECUADO 3.8 mg/24 hours corresponds to 5 mg twice daily of sublingual asenapine and SECUADO 7.6 mg/24 hours corresponds to 10 mg twice daily of sublingual asenapine [see Clinical Pharmacology (12.3)].
2.2 Important Application Instructions
- See the FDA-approved patient labeling (Instructions for Use).
- SECUADO transdermal system is applied once daily. Each SECUADO transdermal system should be worn for 24 hours only. Instruct patients to wear only one SECUADO transdermal system at any time.
- Apply SECUADO to clean, dry, and intact skin at the selected application site. Application sites include: the upper arm, upper back, abdomen, or hip. Apply the transdermal system to a different application site each time a new SECUADO transdermal system is applied.
- Do not cut open the pouch until ready to apply SECUADO and do not use the transdermal system if the individual pouch seal is broken or if it appears to be damaged. Do not cut SECUADO, the whole transdermal system should be applied.
- If the SECUADO transdermal system lifts at the edges, reattach SECUADO by pressing firmly and smoothing down the edges of the system. If SECUADO comes off completely, apply a new SECUADO transdermal system.
- Discard SECUADO by folding the used transdermal system so that the adhesive side sticks to itself and safely discard.
- If irritation or a burning sensation occurs while wearing SECUADO, remove the system and apply a new transdermal system to a new application site [see Warnings and Precautions (5.17)].
- Showering is permitted, but the use of SECUADO during swimming or taking a bath has not been evaluated.
- Do not apply external heat sources (e.g., heating pad) over the SECUADO transdermal system [see Warnings and Precautions (5.16)]. Prolonged application of heat over a SECUADO transdermal system increases plasma concentrations of asenapine [see Clinical Pharmacology (12.3)].
3. Dosage Forms and Strengths
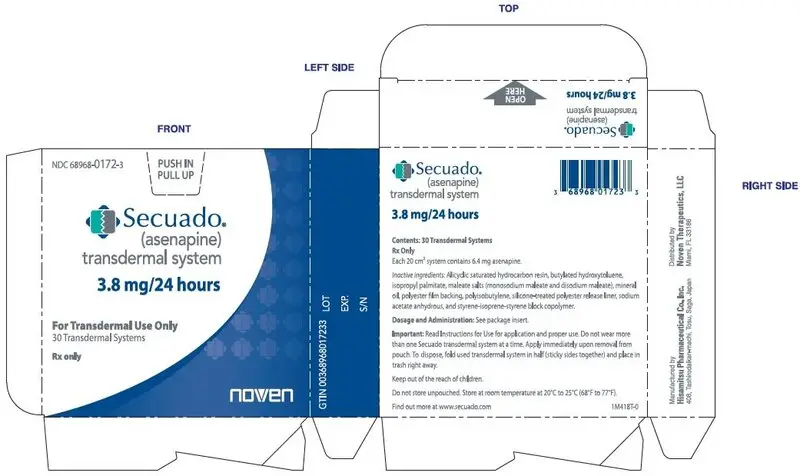
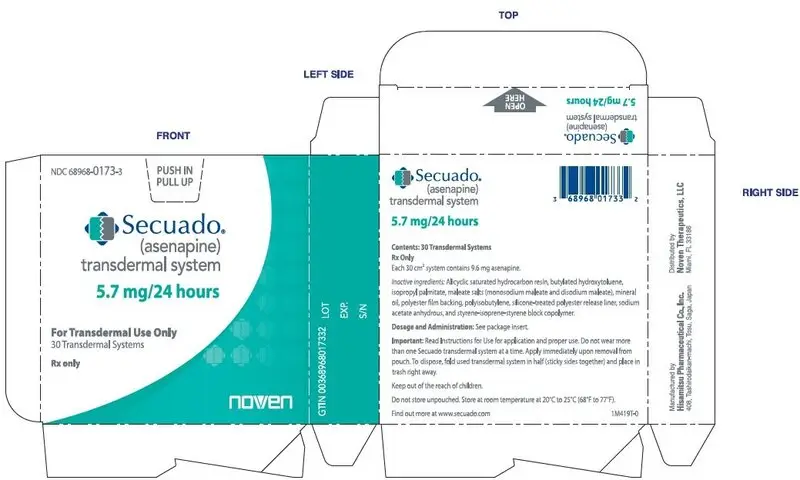
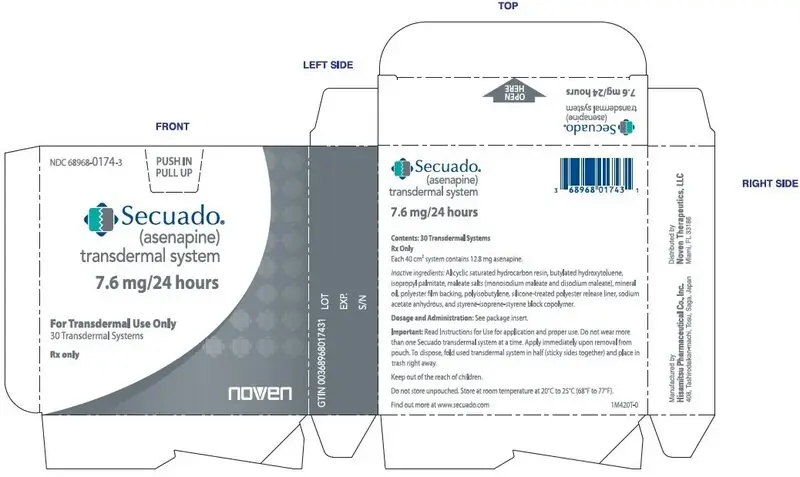
SECUADO (asenapine) transdermal system is a translucent rounded square product available in three dosage strengths:
- 3.8 mg asenapine / 24 hours
- 5.7 mg asenapine / 24 hours
- 7.6 mg asenapine / 24 hours
4. Contraindications
SECUADO is contraindicated in patients with:
- Severe hepatic impairment (Child-Pugh C) [see Specific Populations (8.7), Clinical Pharmacology (12.3)].
- A history of hypersensitivity reactions to asenapine or any components of the transdermal system. Reactions with asenapine have included anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing and rash [see Warnings and Precautions (5.6), Adverse Reactions (6)].
5. Warnings and Precautions
5.1 Increased Mortality in Elderly Patients with Dementia-Related Psychosis
Elderly patients with dementia-related psychosis treated with antipsychotic drugs are at an increased risk of death. Analyses of 17 placebo-controlled trials (modal duration of 10 weeks), largely in patients taking atypical antipsychotic drugs, revealed a risk of death in the drug-treated patients of between 1.6 to 1.7 times that seen in placebo-treated patients. Over the course of a typical 10-week controlled trial, the rate of death in drug-treated patients was about 4.5%, compared to a rate of about 2.6% in the placebo group.
Although the causes of death were varied, most of the deaths appeared to be either cardiovascular (e.g., heart failure, sudden death) or infectious (e.g., pneumonia) in nature. SECUADO is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.2)].
5.2 Cerebrovascular Adverse Reactions, Including Stroke, In Elderly Patients with Dementia-Related Psychosis
In placebo-controlled trials in elderly subjects with dementia, patients randomized to risperidone, aripiprazole, and olanzapine had a higher incidence of stroke and transient ischemic attack, including fatal stroke. SECUADO is not approved for the treatment of patients with dementia-related psychosis [see Warnings and Precautions (5.1)].
5.3 Neuroleptic Malignant Syndrome
A potentially fatal symptom complex sometimes referred to as Neuroleptic Malignant Syndrome (NMS) has been reported in association with administration of antipsychotic drugs. Clinical manifestations of NMS are hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability. Additional signs may include elevated creatine phosphokinase, myoglobinuria (rhabdomyolysis), and acute renal failure. If NMS is suspected, immediately discontinue SECUADO and provide intensive symptomatic treatment and monitoring.
5.4 Tardive Dyskinesia
Tardive dyskinesia, a syndrome of potentially irreversible, involuntary, dyskinetic movements may develop in patients treated with antipsychotic drugs, including SECUADO. The risk appears to be highest among the elderly, especially elderly women, but it is not possible to predict which patients are likely to develop the syndrome. Whether antipsychotic drug products differ in their potential to cause tardive dyskinesia is unknown.
The risk of developing tardive dyskinesia and the likelihood that it will become irreversible increases with the duration of treatment and the cumulative dose. The syndrome can develop after a relatively brief treatment period, even at low doses. It may also occur after discontinuation of treatment.
Tardive dyskinesia may remit, partially or completely, if antipsychotic treatment is discontinued. Antipsychotic treatment itself, however, may suppress (or partially suppress) the signs and symptoms of the syndrome, possibly masking the underlying process. The effect that symptomatic suppression has upon the long-term course of tardive dyskinesia is unknown.
Given these considerations, SECUADO should be prescribed in a manner that is most likely to reduce the risk of tardive dyskinesia. Chronic antipsychotic treatment should generally be reserved for patients: (1) who suffer from a chronic illness that is known to respond to antipsychotic drugs; and (2) for whom alternative, effective, but potentially less harmful treatments are not available or appropriate. In patients who do require chronic treatment, use the lowest dose and the shortest duration of treatment producing a satisfactory clinical response should be sought. Periodically reassess the need for continued treatment.
If signs and symptoms of tardive dyskinesia appear in a patient on SECUADO, drug discontinuation should be considered. However, some patients may require treatment with SECUADO despite the presence of the syndrome.
5.5 Metabolic Changes
Atypical antipsychotic drugs, including SECUADO, have caused metabolic changes, including hyperglycemia, diabetes mellitus, dyslipidemia, and body weight gain. Although all of the drugs in the class to date have been shown to produce some metabolic changes, each drug has its own specific risk profile.
Hyperglycemia and Diabetes Mellitus
Hyperglycemia, in some cases extreme and associated with ketoacidosis or hyperosmolar coma or death, has been reported in patients treated with atypical antipsychotics. There have been reports of hyperglycemia in patients treated with sublingual asenapine. Assess fasting plasma glucose before or soon after initiation of antipsychotic medication, and monitor periodically during long-term treatment.
Reports of hyperglycemia in patients treated with SECUADO were <1% in the placebo-controlled trial. Data from the placebo-controlled schizophrenia trial are presented in Table 1.
| Placebo | SECUADO | ||
|---|---|---|---|
| 3.8 mg/24 hours | 7.6 mg/24 hours | ||
| N* = Number of patients who had assessments at both Baseline and Endpoint. | |||
| Mean Change from Baseline in Fasting Glucose at Endpoint | |||
| Change from Baseline
(mg/dL) (N*) | 0.03 (174) | 3.28 (174) | 3.72 (172) |
| Proportion of Patients with Shifts from Baseline to Endpoint | |||
| Normal to High <100 to ≥ 126 mg/dL(n/N*) | 0% (0/198) | 3.1% (6/196) | 3.0% (6/199) |
| Borderline to High ≥100 and < 126 to ≥126 mg/dL (n/N*) | 2.0% (4/198) | 1.0% (2/196) | 1.0% (2/199) |
In the sublingual asenapine 52-week, double-blind, comparator-controlled trial that included primarily patients with schizophrenia, the mean increase from baseline of fasting glucose was 2.4 mg/dL.
Dyslipidemia
Atypical antipsychotics cause adverse alterations in lipids. Before or soon after initiation of antipsychotic medication, obtain a fasting lipid profile at baseline and monitor periodically during treatment.
Data from the placebo-controlled schizophrenia trial presented in Table 2.
| Placebo | SECUADO | ||
|---|---|---|---|
| 3.8 mg/24 hours | 7.6 mg/24 hours | ||
| N* = Number of patients who had assessments at both Baseline and Endpoint. | |||
| Mean Change from Baseline | |||
| Total Cholesterol
(mg/dL) (N*) | 0.7 (174) | 5.1 (174) | 4.5 (172) |
| LDL
(mg/dL) (N*) | 1.6 (172) | 1.4 (170) | 4.2 (169) |
| HDL
(mg/dL) (N*) | -0.8 (174) | 0.2 (174) | -0.7 (172) |
| Fasting triglycerides
(mg/dL) (N*) | -2.6 (174) | 17.3 (174) | 6.7 (172) |
| Proportion of Patients with Shifts from Baseline to Endpoint(n/N*) | |||
| Total Cholesterol Normal to High <200 to ≥240 mg/dL (n/N*) | 1.0% (2/197) | 2.6% (5/196) | 1.0% (2/199) |
| LDL Normal to High <100 to ≥160 mg/dL (n/N*) | 0.5% (1/195) | 1.0% (2/194) | 0% (0/197) |
| HDL Normal to High ≥40 to <40 mg/dL (n/N*) | 8.1% (16/197) | 10.7% (21/196) | 12.1% (24/199) |
| Fasting Triglycerides Normal to High <150 to ≥200 mg/dL (n/N*) | 1.1% (2/185) | 7.0% (13/185) | 3.2% (6/186) |
In the placebo-controlled schizophrenia trial with SECUADO, the proportion of patients with total cholesterol elevations ≥240 mg/dL (at Endpoint) was 10.7% for patients treated with SECUADO 3.8 mg/24 hours and 13.6% for patients treated with SECUADO 7.6 mg/24 hours versus 10.2 % for placebo-treated patients. The proportion of patients with elevations in triglycerides ≥200 mg/dL (at Endpoint) was 17.8% for SECUADO 3.8 mg/24 hours and 12.4% for SECUADO 7.6 mg/24 hours treated patients versus 10.3% for placebo-treated patients.
Weight Gain
Weight gain has been observed with atypical antipsychotic use, including SECUADO. Monitor weight at baseline and frequently thereafter.
Data on mean changes in body weight and the proportion of subjects meeting a weight gain criterion of ≥7% of body weight from the placebo-controlled schizophrenia trial are presented in Table 3.
| Placebo | SECUADO | ||
|---|---|---|---|
| 3.8 mg/24 hours | 7.6 mg/24 hours | ||
| N* = Number of subjects with data at Endpoint. | |||
| Mean Change from Baseline (kg) (N*) | 0.62 (167) | 2.10 (168) | 2.02 (164) |
| Proportion of Patients with a ≥7% Increase in Body Weight | |||
| % with ≥7% increase in body weight (n/N*) | 3.9% (8/203) | 18.3% (37/202) | 14.3% (29/203) |
In the sublingual asenapine 52-week, double-blind, comparator-controlled adult trial that included primarily patients with schizophrenia, the mean weight gain from baseline was 0.9 kg. The proportion of patients with a ≥7% increase in body weight (at Endpoint) was 14.7%. Table 4 provides the mean weight change from baseline and the proportion of patients with a weight gain of ≥7% categorized by Body Mass Index (BMI) at baseline.
| BMI <23 Sublingual Asenapine N=295 | BMI 23 - ≤27 Sublingual Asenapine N=290 | BMI >27 Sublingual Asenapine N=302 |
|
|---|---|---|---|
| Mean Change from Baseline (kg) | 1.7 | 1 | 0 |
| % with ≥7% increase in body weight | 22% | 13% | 9% |
5.6 Hypersensitivity Reactions
Hypersensitivity reactions have been observed in patients treated with asenapine, including SECUADO. In several cases, these reactions occurred after the first dose. These hypersensitivity reactions included: anaphylaxis, angioedema, hypotension, tachycardia, swollen tongue, dyspnea, wheezing and rash.
5.7 Orthostatic Hypotension, Syncope, and Other Hemodynamic Effects
Atypical antipsychotics cause orthostatic hypotension and syncope. Generally, the risk is greatest during initial dose titration and when increasing the dose.
In the placebo-controlled trial, orthostatic hypotension was reported in 1.5% (3/204) of patients treated with SECUADO 3.8 mg/24 hours and 0% (0/204) of patients treated with SECUADO 7.6 mg/24 hours, compared to <1% (1/206) of patients treated with placebo.
There were no reports of syncope for both doses of SECUADO in the placebo-controlled trial.
During adult pre-marketing clinical trials with sublingual asenapine, including long-term trials without comparison to placebo, syncope was reported in 0.6% (11/1953) of patients treated with sublingual asenapine.
Orthostatic vital signs should be monitored in patients who are vulnerable to hypotension (elderly patients, patients with dehydration, hypovolemia, concomitant treatment with antihypertensive medications), patients with known cardiovascular disease (history of myocardial infarction or ischemic heart disease, heart failure, or conduction abnormalities), and patients with cerebrovascular disease. SECUADO should be used cautiously when treating patients who receive treatment with other drugs that can induce hypotension, bradycardia, respiratory or central nervous system depression [see Drug Interactions (7.1)]. Monitoring of orthostatic vital signs should be considered in all such patients, and a dose reduction should be considered if hypotension occurs.
5.8 Falls
SECUADO may cause somnolence, postural hypotension, motor and sensory instability, which may lead to falls and, consequently, fractures or other injuries. For patients with diseases, conditions, or medications that could exacerbate these effects, complete fall risk assessments when initiating antipsychotic treatment and recurrently for patients on long-term antipsychotic therapy.
5.9 Leukopenia, Neutropenia, and Agranulocytosis
In clinical trials and/or postmarketing experience, events of leukopenia and neutropenia have been reported temporally related to antipsychotic agents, including asenapine. Agranulocytosis (including fatal cases) has also been reported with other agents in the class.
Possible risk factors for leukopenia/neutropenia include pre-existing low white blood cell count (WBC) or absolute neutrophil count (ANC) and history of drug-induced leukopenia/neutropenia. In patients with pre-existing low WBC or ANC or history of drug-induced leukopenia or neutropenia, perform a complete blood count (CBC) frequently during the first few months of therapy. In such patients, consider discontinuation of SECUADO at the first sign of a clinically significant decline in WBC in the absence of other causative factors.
Monitor patients with clinically significant neutropenia for fever or other symptoms or signs of infection and treat promptly if such symptoms or signs occur. Discontinue SECUADO in patients with severe neutropenia (absolute neutrophil count <1000/mm3) and follow their WBC until recovery.
5.10 QT Prolongation
The effects of sublingual asenapine on the QT/QTc interval were evaluated in a dedicated adult QT study. This trial involved sublingual asenapine doses of 5 mg, 10 mg, 15 mg, and 20 mg twice daily, and placebo, and was conducted in 151 clinically stable patients with schizophrenia, with electrocardiographic assessments throughout the dosing interval at baseline and steady state. At these doses, sublingual asenapine was associated with increases in QTc interval ranging from 2 to 5 msec compared to placebo. No patients treated with sublingual asenapine experienced QTc increases ≥60 msec from baseline measurements, nor did any patient experience a QTc of ≥500 msec.
Electrocardiogram (ECG) measurements were taken at various time points during the SECUADO clinical trial (3.8 mg/24 hours and 7.6 mg/24 hours doses). In the placebo-controlled trial, there were no reports of QT prolongations exceeding 500 msec for SECUADO and placebo.
There were no reports of Torsades de Pointes or any other adverse reactions associated with delayed ventricular repolarization with sublingual asenapine or with SECUADO.
The use of SECUADO should be avoided in combination with other drugs known to prolong QTc including Class 1A antiarrhythmics (e.g., quinidine, procainamide) or Class 3 antiarrhythmics (e.g., amiodarone, sotalol), antipsychotic medications (e.g., ziprasidone, chlorpromazine, thioridazine), and antibiotics (e.g., gatifloxacin, moxifloxacin). SECUADO should also be avoided in patients with a history of cardiac arrhythmias and in other circumstances that may increase the risk of the occurrence of torsade de pointes and/or sudden death in association with the use of drugs that prolong the QTc interval, including bradycardia; hypokalemia or hypomagnesemia; and presence of congenital prolongation of the QT interval.
5.11 Hyperprolactinemia
Like other drugs that antagonize dopamine D2 receptors, SECUADO can elevate prolactin levels, and the elevation can persist during chronic administration. Hyperprolactinemia may suppress hypothalamic GnRH, resulting in reduced pituitary gonadotropin secretion. This, in turn, may inhibit reproductive function by impairing gonadal steroidogenesis in both female and male patients. Galactorrhea, amenorrhea, gynecomastia, and impotence have been reported in patients receiving prolactin-elevating compounds. Long-standing hyperprolactinemia when associated with hypogonadism may lead to decreased bone density in both female and male subjects.
In the SECUADO placebo-controlled trial, galactorrhea, amenorrhea, gynecomastia, and impotence were not reported for patients treated with SECUADO or placebo [see Adverse Reactions (6.1)].
In sublingual asenapine adult pre-marketing clinical trials, the incidences of adverse events related to abnormal prolactin levels were 0.4% versus 0% for placebo.
Tissue culture experiments indicate that approximately one-third of human breast cancers are prolactin-dependent in vitro, a factor of potential importance if the prescription of these drugs is considered in a patient with previously-detected breast cancer. Neither clinical studies nor epidemiologic studies conducted to date have shown an association between chronic administration of this class of drugs and tumorigenesis in humans, but the available evidence is too limited to be conclusive.
5.12 Seizures
In the SECUADO placebo-controlled trial, there were no reports of seizures in adult patients treated with doses of 3.8 mg/24 hours and 7.6 mg/24 hours of SECUADO.
During adult pre-marketing clinical trials with sublingual asenapine, including long-term trials without comparison to placebo, seizures were reported in 0.3% (5/1953) of patients treated with sublingual asenapine.
As with other antipsychotic drugs, SECUADO should be used with caution in patients with a history of seizures or with conditions that potentially lower the seizure threshold. Conditions that lower the seizure threshold may be more prevalent in patients 65 years or older.
5.13 Potential for Cognitive and Motor Impairment
SECUADO, like other antipsychotics, has the potential to impair judgment, thinking or motor skills. Patients should be cautioned about operating hazardous machinery, including motor vehicles, until they are reasonably certain that SECUADO therapy does not affect them adversely.
Somnolence was reported in patients treated with SECUADO. In the short-term, fixed-dose, placebo-controlled schizophrenia adult trial, somnolence was reported in 4.4% (9/204) of patients on SECUADO 3.8 mg/24 hours and in 3.4% (7/204) of patients on SECUADO 7.6 mg/24 hours compared to 1.5% (3/206) of placebo patients. There were no reports of somnolence that led to discontinuation in the placebo-controlled trial.
During adult pre-marketing clinical trials with sublingual asenapine, including long-term trials without comparison to placebo, somnolence was reported in 18% (358/1953) of patients treated with sublingual asenapine.
5.14 Body Temperature Regulation
Atypical antipsychotics may disrupt the body’s ability to reduce core body temperature.
Strenuous exercise, exposure to extreme heat, dehydration, and anticholinergic medications may contribute to an elevation in core body temperature; use SECUADO with caution in patients who may experience these conditions.
5.15 Dysphagia
Esophageal dysmotility and aspiration have been associated with antipsychotic drug use. There were no reports of dysphagia with SECUADO; however, dysphagia has been reported with sublingual asenapine. SECUADO and other antipsychotic drugs should be used cautiously in patients at risk for aspiration.
5.16 External Heat
When heat is applied to SECUADO after application, both the rate and extent of absorption are increased. After application of a heating pad, asenapine exposure (partial AUC0-8) was about 3.9 times greater than without heating pad application [see Clinical Pharmacology (12.3)]. Advise patients to avoid exposing SECUADO to direct external heat sources such as hair dryers, heating pads, electric blankets, heated water beds, etc., while wearing SECUADO.
5.17 Application Site Reactions
Local skin reactions, such as irritation, were reported with SECUADO. During wear time or immediately after removal of SECUADO, the skin at the site of application may develop erythema, pruritus, papules, discomfort, pain, edema, or irritation. In the short-term, fixed-dose, placebo-controlled schizophrenia adult trial, application site reactions were reported in 15.2% (31/204) of patients on SECUADO 3.8 mg/24 hours and in 13.7% (28/204) of patients on SECUADO 7.6 mg/24 hours compared to 3.9% (8/206) of placebo patients. The most common application site reaction was erythema, which was reported in 9.3% (19/204) of patients on SECUADO 3.8 mg/24 hours and in 9.8% (20/204) of patients on SECUADO 7.6 mg/24 hours compared to 1.5% (3/206) of placebo patients. Another common application site reaction was pruritus, which was reported in 4.9% (10/204) of patients on SECUADO 3.8 mg/24 hours and in 3.9% (8/204) of patients on SECUADO 7.6 mg/24 hours compared to 1.9% (4/206) of placebo patients. One patient developed application site discoloration (hyperpigmentation) at multiple application sites that persisted for at least several weeks after discontinuing SECUADO treatment. Application site reactions occurred more frequently in Black or African American patients compared to Caucasians. Inform patients of these potential reactions and that increased skin irritation may occur with SECUADO if applied for a longer period than instructed or if the same application site is used repeatedly. Instruct patients to select a different application site each day to minimize skin reactions.
6. Adverse Reactions/Side Effects
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Use in Elderly Patients with Dementia-Related Psychosis [see Warning and Precautions (5.1)]
- Cerebrovascular Adverse Reactions, Including Stroke, in Elderly Patients with Dementia-Related Psychosis [see Warnings and Precautions (5.2)]
- Neuroleptic Malignant Syndrome [see Warnings and Precautions (5.3)]
- Tardive Dyskinesia [see Warnings and Precautions (5.4)]
- Metabolic Changes [see Warnings and Precautions (5.5)]
- Hypersensitivity Reactions [see Contraindications(4),Warnings and Precautions (5.6) and Patient Counseling Information (17)]
- Orthostatic Hypotension, Syncope, and other Hemodynamic Effects [see Warnings and Precautions (5.7)]
- Falls [see Warnings and Precautions (5.8)]
- Leukopenia, Neutropenia, and Agranulocytosis [see Warnings and Precautions (5.9)]
- QT Interval Prolongation [see Warnings and Precautions (5.10)]
- Hyperprolactinemia [see Warnings and Precautions (5.11)]
- Seizures [see Warnings and Precautions (5.12)]
- Potential for Cognitive and Motor Impairment [see Warnings and Precautions (5.13)]
- Body Temperature Regulation [see Warnings and Precautions (5.14)]
- Dysphagia [see Warnings and Precautions (5.15)]
- External Heat [see Warnings and Precautions (5.16)]
- Application Site Reactions [see Warnings and Precautions (5.17)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The safety of SECUADO was evaluated in a total of 315 adult patients diagnosed with schizophrenia who were exposed to SECUADO for up to 6 weeks in a placebo-controlled trial.
Adverse Reactions Leading to Discontinuation of Treatment
A total of 4.9% (10/204) patients treated with SECUADO 3.8 mg/24 hours, 7.8% (16/204) patients treated with SECUADO 7.6 mg/24 hours, and 6.8% (14/206) patients on placebo discontinued due to adverse reactions in the placebo-controlled trial. The adverse reaction that most commonly led to discontinuation among SECUADO-treated patients in this trial was akathisia, which led to discontinuation in no (0/204) patients treated with SECUADO 3.8 mg/24 hours, 1.5% (3/204) patients treated with SECUADO 7.6 mg/24 hours, and 0.5% (1/206) patients on placebo.
Commonly Observed Adverse Reactions
The most common adverse reactions (≥5% and at least twice the rate of placebo) reported in adult patients with schizophrenia treated with SECUADO in the placebo-controlled trial were extrapyramidal disorder, application site reaction and weight gain.
Adverse Reactions Occurring at an Incidence of 2% or More in SECUADO-Treated Patients.
Adverse reactions associated with the use of SECUADO (incidence of ≥2%, rounded to the nearest percent, and SECUADO incidence greater than placebo) that occurred during the placebo-controlled trial are shown in Table 5.
| * The following terms were combined:
Application site reactions includes application site dermatitis, discoloration, discomfort, dryness, edema, erythema, exfoliation, induration, irritation, pain, papules, pruritus, and reaction. Blood glucose increased includes blood glucose increased, blood insulin increased, glycosylated hemoglobin increased, hyperglycemia, Type 2 diabetes mellitus, diabetes mellitus, and hyperinsulinemia. Hepatic enzyme increased includes hepatic enzyme increased, alanine aminotransferase increased, aspartate aminotransferase increased, and gamma-glutamyltransferase increased. Extrapyramidal symptoms includes dyskinesia, dystonia, extrapyramidal disorder, parkinsonism. tardive dyskinesia, muscle spasm, and musculoskeletal stiffness. Somnolence includes somnolence, sedation, lethargy, and hypersomnia. Hypertension includes hypertension, blood pressure increased, diastolic hypertension, and hypertensive crisis. |
|||
| System Organ Class/ Preferred Term | Placebo N = 206 (%) | SECUADO | |
|---|---|---|---|
| 3.8 mg/24 hours N = 204 (%) | 7.6 mg/24 hours N = 204 (%) |
||
| Gastrointestinal disorders | |||
| Constipation | 4 | 5 | 4 |
| Dyspepsia | 1 | 1 | 3 |
| Diarrhea | 1 | 3 | 1 |
| General Disorders | |||
| Application Site Reactions* | 4 | 15 | 14 |
| Investigations | |||
| Blood glucose increased* | 1 | 3 | 1 |
| Weight Increased | 2 | 4 | 6 |
| Hepatic enzyme increased* | 0 | 2 | 2 |
| Infections and Infestations | |||
| Nasopharyngitis | 2 | 3 | 1 |
| Upper respiratory tract infection | 2 | 3 | 1 |
| Metabolism and nutrition disorders | |||
| Increased appetite | 0 | 3 | 1 |
| Nervous System Disorders | |||
| Headache | 6 | 9 | 9 |
| Extrapyramidal symptoms* | 2 | 8 | 13 |
| Akathisia | 2 | 4 | 4 |
| Somnolence* | 1 | 4 | 3 |
| Dystonia | 0 | 1 | 3 |
| Vascular Disorders | |||
| Hypertension* | 1 | 2 | 2 |
Dose-Related Adverse Reactions: In the placebo-controlled schizophrenia trial, the incidence of an extrapyramidal disorder and weight increased appear to be dose-related (see Table 5).
Dystonia:
Symptoms of dystonia, prolonged abnormal contractions of muscle groups, may occur in susceptible individuals during the first few days of treatment. Dystonic symptoms include: spasm of the neck muscles, sometimes progressing to tightness of the throat, swallowing difficulty, difficulty breathing, and/or protrusion of the tongue. While these symptoms can occur at low doses, they occur more frequently and with greater severity with high potency and at higher doses of first generation antipsychotic drugs. An elevated risk of acute dystonia is observed in males and younger age groups [Clinical Pharmacology (12.3)].
Extrapyramidal Symptoms:
In the short-term, placebo-controlled schizophrenia adult trial, data were objectively collected on the Simpson Angus Rating Scale for extrapyramidal symptoms (EPS), the Barnes Akathisia Scale (for akathisia) and the Assessments of Involuntary Movement Scales (for dyskinesias). The mean change from baseline for the SECUADO 3.8 mg/24 hours or 7.6 mg/24 hours treated group was similar to placebo in each of the rating scale scores.
In the short-term, placebo-controlled schizophrenia adult trial, the incidence of reported extrapyramidal disorder events, excluding events related to akathisia, was 7.8% for patients treated with SECUADO 3.8 mg/24 hours, 12.8% for patients treated with SECUADO 7.6 mg/24 hours and 2.4% for placebo-treated patients; and the incidence of akathisia-related events was 3.9% for patients treated with SECUADO 3.8 mg/24 hours, 4.4% for patients treated with SECUADO 7.6 mg/24 hours and 2.4% for placebo-treated patients.
Laboratory Test Abnormalities:
Transaminases: Transient elevations in serum transaminases (primarily ALT) were more common in SECUADO-treated patients. The mean increase in ALT levels for SECUADO-treated patients was 6.0 units/L and 3.8 units/L for the SECUADO 3.8 mg/24 hours and 7.6 mg/24 hours treated groups, respectively, compared to a decrease of 1.1 units/L for placebo-treated patients. The proportion of patients with ALT elevations ≥3 times upper limit of normal (ULN) (at any time) was 1.6% and 3.1% for patients treated with SECUADO 3.8 mg/24 hours and 7.6 mg/24 hours, respectively, and 0% for placebo-treated patients.
In a 52-week, double-blind, comparator-controlled trial that included primarily adult patients with schizophrenia, the mean increase from baseline of ALT was 1.7 units/L for sublingual asenapine.
Prolactin: The proportion of patients with prolactin elevations ≥4 times ULN (at Endpoint) were 0.0% and 1.3% for patients treated with SECUADO 3.8 mg/24 hours and 7.6 mg/24 hours, respectively, as compared to 2.4% for placebo-treated patients in the short-term placebo-controlled trial.
In a long-term (52-week), double-blind, comparator-controlled adult trial that included primarily patients with schizophrenia, the mean decrease in prolactin from baseline for sublingual asenapine-treated patients was 26.9 ng/mL.
Creatine Kinase (CK): The proportion of adult patients with CK elevations ≥3 times ULN at any time were 1.6% and 2.1% for patients treated with SECUADO 3.8 mg/24 hours and 7.6 mg/24 hours, respectively, as compared to 1.5% for placebo-treated patients in the short-term, placebo-controlled trial. The clinical relevance of this finding is unknown.
Other Adverse Reactions Observed During the Premarketing Evaluation of SECUADO
Other adverse reactions (<2% frequency) within the 6-week placebo-controlled trial in patients with schizophrenia are listed below. The reactions listed are those that could be of clinical importance, as well as reactions that are plausibly drug-related on pharmacologic or other grounds. Reactions that appear elsewhere in the SECUADO label are not included.
Gastrointestinal disorders: vomiting, dry mouth
General disorders and administration site conditions: asthenia
Musculoskeletal and connective tissue disorders: myalgia
Other Adverse Reactions Reported in Clinical Trials with Sublingual Asenapine
Following is a list of MedDRA terms that reflect adverse reactions reported by patients treated with sublingual asenapine at multiple doses of ≥5 mg twice daily during any phase of a trial within the database of adult patients. The reactions listed are those that could be of clinical importance, as well as reactions that are plausibly drug-related on pharmacologic or other grounds. Reactions already listed for adult patients in other parts of Adverse Reactions (6), or those considered in Contraindications (4), Warnings and Precautions (5) or Overdosage (10) are not included. Reactions are further categorized by MedDRA system organ class and listed in order of decreasing frequency according to the following definitions: those occurring in at least 1/100 patients (frequent) (only those not already listed in the tabulated results from placebo-controlled trials appear in this listing); those occurring in 1/100 to 1/1000 patients (infrequent); and those occurring in fewer than 1/1000 patients (rare).
Blood and lymphatic disorders: infrequent: anemia; rare: thrombocytopenia
Cardiac disorders: infrequent: temporary bundle branch block
Eye disorders: infrequent: accommodation disorder
Gastrointestinal disorders: infrequent: swollen tongue
General disorders: rare: idiosyncratic drug reaction
Investigations: infrequent: hyponatremia
Nervous system disorders: infrequent: dysarthria
6.2 Postmarketing Experience
The following adverse reactions have been identified during post-approval use of sublingual asenapine and are possible with SECUADO treatment. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to establish a causal relationship to drug exposure.
- Choking has been reported by patients, some of whom may have also experienced oropharyngeal muscular dysfunction.
7. Drug Interactions
7.1 Drugs Having Clinically Important Drug Interactions with SECUADO
| Antihypertensive Drugs | |
| Clinical Implication | Because of its α1-adrenergic antagonism with potential for inducing hypotension, SECUADO may enhance the effects of certain antihypertensive agents [see Warnings and Precautions (5.7)]. |
| Prevention or Management: | Monitor blood pressure and adjust dosage of antihypertensive drug accordingly. |
| Examples: | Diuretics, ACE Inhibitors, Angiotensin Receptor Blockers, and Beta-Blockers, Alpha-blockers |
| Strong CYP1A2 Inhibitors | |
| Clinical Implication | Asenapine is metabolized by CYP1A2. Concomitant use of SECUADO with a CYP1A2 inhibitor increases AUC and Cmax of asenapine [see Clinical Pharmacology (12.3)]. |
| Prevention or Management: | Dosage reduction for SECUADO based on clinical response may be necessary. |
| Examples: | Fluvoxamine, ciprofloxacin, enoxacin |
| CYP2D6 substrates and inhibitors | |
| Clinical Implication | Asenapine may enhance the inhibitory effects of paroxetine on its own metabolism by CYP2D6. Concomitant use of SECUADO with paroxetine increases paroxetine AUC and Cmax[see Clinical Pharmacology (12.3)]. |
| Prevention or Management: | Reduce paroxetine dose by half when paroxetine is used in combination with SECUADO. |
| Examples: | Paroxetine |
8. Use In Specific Populations
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to atypical antipsychotics, including SECUADO, during pregnancy. For more information contact the National Pregnancy Registry for Atypical Antipsychotics at 1-866-961-2388 or visit http://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/.
Risk Summary
Neonates exposed to antipsychotic drugs during the third trimester of pregnancy are at risk for extrapyramidal and/or withdrawal symptoms. Studies have not been conducted with SECUADO in pregnant women. There are no available human data informing the drug-associated risk. The background risk of major birth defects and miscarriage for the indicated populations are unknown. However, the background risk in the U.S. general population of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies. No teratogenicity was observed in animal reproduction studies with intravenous administration of asenapine to rats and rabbits during organogenesis at doses 0.7 and 0.4 times, respectively, the maximum recommended human dose (MRHD) of 10 mg of sublingual asenapine twice daily and 1.1 and 0.66 times, respectively, the MRHD of 12.8 mg of transdermal asenapine daily. In a pre-and post-natal study in rats, intravenous administration of asenapine at doses up to 0.7 times the MRHD of 10 mg of sublingual asenapine twice daily produced increases in post-implantation loss and early pup deaths, and decreases in subsequent pup survival and weight gain [see Data]. These doses are up to 1.1 times the MRHD of 12.8 mg transdermal asenapine daily. Advise pregnant women of the potential risk to a fetus.
Clinical Considerations
Fetal/Neonatal Adverse Reactions
Extrapyramidal and/or withdrawal symptoms, including agitation, hypertonia, hypotonia, tremor, somnolence, respiratory distress and feeding disorder have been reported in neonates who were exposed to antipsychotic drugs during the third trimester of pregnancy. These symptoms have varied in severity. Some neonates recovered within hours or days without specific treatment; others required prolonged hospitalization. Monitor neonates for extrapyramidal and/or withdrawal symptoms and manage symptoms appropriately.
Animal Data
In animal studies, asenapine increased post-implantation loss and decreased pup weight and survival at doses similar to or less than recommended clinical doses. In these studies, there was no increase in the incidence of structural abnormalities caused by asenapine.
Asenapine was not teratogenic in reproduction studies in rats and rabbits at intravenous doses up to 1.5 mg/kg in rats and 0.44 mg/kg in rabbits administered during organogenesis. These doses are 0.7 and 0.4 times, respectively, MRHD of 10 mg of sublingual asenapine twice daily and 1.1 and 0.66 times, respectively, the MRHD of 12.8 mg transdermal asenapine daily. Plasma levels of asenapine were measured in the rabbit study, and the area under the curve (AUC) at the highest dose tested was 2 times that in humans receiving the MRHD of 10 mg of sublingual asenapine twice daily.
In a study in which rats were treated from day 6 of gestation through day 21 postpartum with intravenous doses of asenapine of 0.3, 0.9, and 1.5 mg/kg/day (0.15, 0.44, and 0.7 times the MRHD of 10 mg of sublingual asenapine twice daily and 0.22, 0.68 and 1.13 times the MRHD of 12.8 mg transdermal asenapine daily), increases in post-implantation loss and early pup deaths were seen at all doses, and decreases in subsequent pup survival and weight gain were seen at the two higher doses. A cross-fostering study indicated that the decreases in pup survival were largely due to prenatal drug effects. Increases in post-implantation loss and decreases in pup weight and survival were also seen when pregnant rats were dosed orally with asenapine.
8.2 Lactation
Risk Summary
Lactation studies have not been conducted to assess the presence of asenapine in human milk, the effects of asenapine on the breastfed infant, or the effects of asenapine on milk production. Asenapine is excreted in rat milk. The development and health benefits of breastfeeding should be considered along with the mother's clinical need for SECUADO and any potential adverse effects on the breastfed infant from SECUADO or from the underlying maternal condition.
8.4 Pediatric Use
Safety and effectiveness of SECUADO in pediatric patients have not been established.
Efficacy of sublingual asenapine was not demonstrated in an 8-week, placebo-controlled, double-blind trial, in 306 adolescent patients aged 12 to 17 years with schizophrenia at doses of 2.5 and 5 mg twice daily. The most common adverse reactions (proportion of patients equal or greater than 5% and at least twice placebo) reported were somnolence, akathisia, dizziness, and oral hypoesthesia or paresthesia. The proportion of patients with an equal or greater than 7% increase in body weight at endpoint compared to baseline for placebo, sublingual asenapine 2.5 mg twice daily, and sublingual asenapine 5 mg twice daily was 3%, 10%, and 10%, respectively. No new major safety findings were reported from a 26-week, open-label, uncontrolled safety trial in pediatric patients with schizophrenia treated with sublingual asenapine.
Juvenile Animal Data
Subcutaneous administration of asenapine to juvenile rats for 56 days from day 14 of age to day 69 of age at 0.4, 1.2, and 3.2 mg/kg/day (0.2, 0.6 and 1.5 times the maximum recommended human dose of 10 mg twice daily given sublingually on a mg/m2 basis) resulted in significant reduction in body weight gain in animals of both sexes at all dose levels from the start of dosing until weaning. Body weight gain remained reduced in males to the end of treatment, however, recovery was observed once treatment ended. Neurobehavioral assessment indicated increased motor activity in animals at all dose levels following the completion of treatment, with the evidence of recovery in males. There was no recovery after the end of treatment in female activity pattern as late as day 30 following the completion of treatment (last retesting). Therefore, a No Observed Adverse Effect Level (NOAEL) for the juvenile animal toxicity of asenapine could not be determined. There were no treatment-related effects on the startle response, learning/memory, organ weights, microscopic evaluations of the brain and, reproductive performance (except for minimally reduced conception rate and fertility index in males and females administered 1.2 and 3.2 mg/kg/day).
8.5 Geriatric Use
The SECUADO placebo-controlled trial for the treatment of schizophrenia did not include sufficient numbers of patients aged 65 and over to determine whether or not they respond differently than younger patients. Of the approximately 614 patients in placebo-controlled study of SECUADO, 1.6% (10) were 65 years of age or over.
Multiple factors that might increase the pharmacodynamic response to SECUADO, causing poorer tolerance or orthostasis, could be present in elderly patients, and these patients should be monitored carefully. Based on a pharmacokinetic study in elderly patients with sublingual asenapine, dosage adjustments are not recommended based on age alone [see Clinical Pharmacology (12.3)].
Elderly patients with dementia-related psychosis treated with SECUADO are at an increased risk of death compared to placebo. SECUADO is not approved for the treatment of patients with dementia-related psychosis [see Warning and Precautions (5.1, 5.2)].
8.6 Renal Impairment
No dosage adjustment for SECUADO is required on the basis of a patient’s renal function (mild to severe renal impairment, glomerular filtration rate between 15 and 90 mL/minute). The exposure of asenapine was similar among subjects with varying degrees of renal impairment and subjects with normal renal function [see Clinical Pharmacology (12.3)]. The effect of renal function on the excretion of other metabolites and the effect of dialysis on the pharmacokinetics of asenapine has not been studied.
8.7 Hepatic Impairment
SECUADO is contraindicated in patients with severe hepatic impairment (Child-Pugh C) because asenapine exposure is 7-fold higher in subjects with severe hepatic impairment than the exposure observed in subjects with normal hepatic function.
No dosage adjustment for SECUADO is required in patients with mild to moderate hepatic impairment (Child-Pugh A and B) because asenapine exposure is similar to that in subjects with normal hepatic function [see Contraindications (4) and Clinical Pharmacology (12.3)].
9. Drug Abuse and Dependence
9.2 Abuse
SECUADO has not been systematically studied in animals or humans for its abuse potential or its ability to induce tolerance or physical dependence. Thus, it is not possible to predict the extent to which a CNS-active drug will be misused, diverted and/or abused once it is marketed. Patients should be evaluated carefully for a history of drug abuse, and such patients should be observed carefully for signs that they are misusing or abusing SECUADO (e.g., drug-seeking behavior, increases in dose).
10. Overdosage
Human Experience: In the placebo-controlled trial in adults for SECUADO, there were no reports of accidental or intentional acute overdosage of SECUADO.
In adult clinical studies involving more than 3350 patients and/or healthy subjects for sublingual asenapine, accidental or intentional acute overdosage of sublingual asenapine was identified in 3 patients. Among these few reported cases of overdose, the highest estimated ingestion of sublingual asenapine was 400 mg. Reported adverse reactions at the highest dosage included agitation and confusion.
Management of Overdosage: There is no specific antidote to SECUADO. The possibility of multiple drug involvement should be considered. An electrocardiogram should be obtained and management of overdose should concentrate on supportive therapy, maintaining an adequate airway, oxygenation and ventilation, and management of symptoms. Consult a Certified Poison Control Center at 1 800-222-1222 for up to date information on the management of overdosage.
Hypotension and circulatory collapse should be treated with appropriate measures, such as intravenous fluids and/or sympathomimetic agents (epinephrine and dopamine should not be used, since beta stimulation may worsen hypotension in the setting of SECUADO-induced alpha blockade). In case of severe extrapyramidal symptoms, anticholinergic medication should be administered. Close medical supervision and monitoring should continue until the patient recovers.
11. Secuado Film Description
SECUADO transdermal system contains asenapine, an atypical antipsychotic.
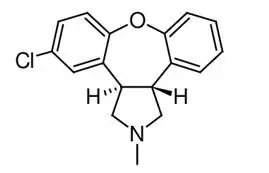
Asenapine belongs to the class dibenzo-oxepino pyrroles. The chemical name is trans-5-chloro-2-methyl-2,3,3a,12b-tetrahydro-1H-dibenz[2,3:6,7] oxepino [4,5-c] pyrrole. Its molecular formula is C17H16Cl NO and its molecular weight is 285.8 g/mol. The chemical structure is:
SECUADO is for transdermal administration and is provided in three strengths: 3.8 mg, 5.7 mg or 7.6 mg asenapine every 24 hours (Table 7). The composition of the transdermal systems per unit area is identical. Inactive ingredients include alicyclic saturated hydrocarbon resin, butylated hydroxytoluene, isopropyl palmitate, maleate salts (monosodium maleate and disodium maleate), mineral oil, polyester film backing, polyisobutylene, silicone-treated polyester release liner, sodium acetate anhydrous, and styrene-isoprene-styrene block copolymer.
| Dosage Strength (Asenapine) | Total Asenapine Content per Transdermal System | Transdermal System Size |
| 3.8 mg/24 hours | 6.4 mg | 20 cm2 |
| 5.7 mg/24 hours | 9.6 mg | 30 cm2 |
| 7.6 mg/24 hours | 12.8 mg | 40 cm2 |
12. Secuado Film - Clinical Pharmacology
12.1 Mechanism of Action
The mechanism of action of asenapine in schizophrenia is unclear. However, it’s efficacy in schizophrenia could be mediated through a combination of antagonist activity at D2 and 5-HT2A receptors.
12.2 Pharmacodynamics
Asenapine exhibits high affinity for serotonin 5-HT1A, 5-HT1B, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT5A, 5-HT6 and 5-HT7 receptors (Ki values of 2.5, 2.7, 0.07, 0.18, 0.03, 1.6, 0.25, and 0.11nM, respectively), dopamine D2A, D2B, D3, D4, and D1 receptors (Ki values of 1.3, 1.4, 0.42, 1.1, and 1.4 nM, respectively), adrenergic α1A, α2A, α2B, and α2C receptors (Ki values of 1.2, 1.2, 0.33 and 1.2 nM, respectively), and histamine H1 receptors (Ki value 1.0 nM), and moderate affinity for H2 receptors (Ki value of 6.2 nM). In in vitro assays asenapine acts as an antagonist at these receptors. Asenapine has no appreciable affinity for muscarinic cholinergic receptors (e.g., Ki value of 8128 nM for M1).
12.3 Pharmacokinetics
SECUADO has a different pharmacokinetic profile compared to sublingual asenapine. Maximum asenapine concentrations are typically reached between 12 and 24 hours, with sustained concentrations during wear time (24 hours). Following SECUADO removal, the apparent elimination half-life is approximately 30 hours.
Absorption
On average, approximately 60% of the asenapine is released from the transdermal system over 24 hours. Inter-individual variability for SECUADO as coefficient of variation (%CV) for the steady-state asenapine Cmax,ss, Cmin,ss, and AUC0-tau,ss was generally about 20% to 30%.
Asenapine PK at steady-state is dose-proportional in the dose range of 3.8 mg/24 hours to 7.6 mg/24 hours following application of SECUADO. Steady-state plasma concentrations are achieved in about 72 hours after the first application of SECUADO. Peak to trough ratio is 1.5.
Based on relative bioavailability and established dose proportionality, AUC for 3.8 mg/24 hours and 7.6 mg/24 hours was considered to be similar to that for sublingual asenapine 5 mg twice daily and 10 mg twice daily, respectively.
There is no effect on asenapine PK with regards to the application site (upper arm, upper back, abdomen, and hip area).
Application of a heating pad on SECUADO for 8 hours led to a faster absorption rate (median tmax about 8 hours) as compared with SECUADO without a heating pad (median tmax about 16 hours). Mean asenapine exposure, calculated as partial AUC0-8, following SECUADO application, was about 3.9 times greater than that without a heating pad, indicating the apparent heat effect in absorption during the time period of heating pad application.
Distribution
Asenapine is rapidly distributed and has a large volume of distribution (approximately 20-25 L/kg), indicating extensive extravascular distribution. Asenapine is highly bound (95%) to plasma proteins, including albumin and α1-acid glycoprotein.
Elimination
Asenapine is a high clearance drug with a clearance after intravenous administration of 52 L/h. In this circumstance, hepatic clearance is influenced primarily by changes in liver blood flow rather than by changes in the intrinsic clearance, i.e., the metabolizing enzymatic activity
Metabolism
Direct glucuronidation by UGT1A4 and oxidative metabolism by cytochrome P450 isoenzymes (predominantly CYP1A2) are the primary metabolic pathways for asenapine.
Excretion
After administration of a single dose of [14C]-labeled asenapine, about 90% of the dose was recovered; approximately 50% was recovered in urine, and 40% recovered in feces. About 50% of the circulating species in plasma have been identified. The predominant species was asenapine N -glucuronide; others included N-desmethylasenapine, N-desmethylasenapine N-carbamoyl glucuronide, and unchanged asenapine in smaller amounts. Pharmacological activity is primarily due to the parent drug.
Special Populations
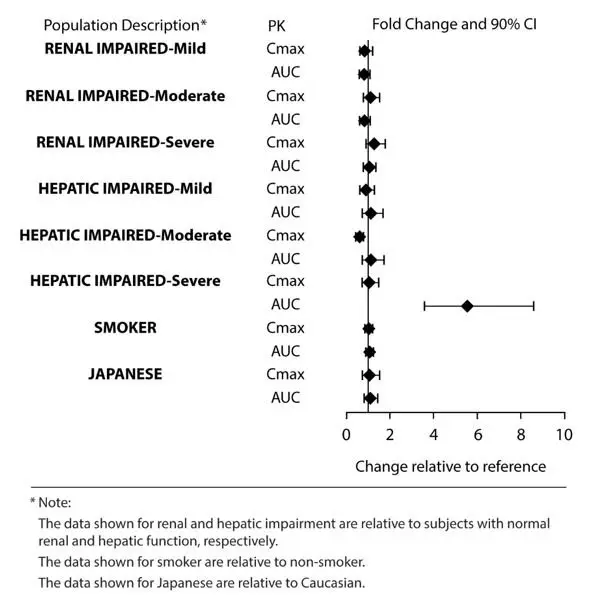
Exposures of asenapine in special populations for sublingual asenapine are summarized in Figure 1.
Based on population pharmacokinetic analysis for sublingual asenapine, no effects of sex, race, ethnicity (Japanese versus Caucasian), BMI, and smoking status on asenapine exposure were observed. Exposure in elderly patients is 30-40% higher as compared to adults.
Figure 1: Effect of Intrinsic Factors on Sublingual Asenapine Pharmacokinetics
Drug Interaction Studies
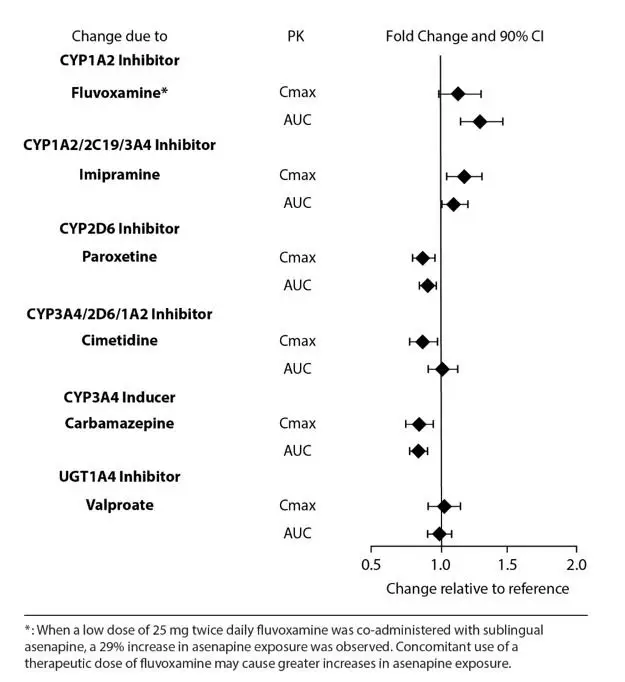
Effects of other drugs on the exposure of asenapine are summarized in Figure 2. Marginal increase of asenapine exposure was observed when sublingual asenapine is used with fluvoxamine at 25 mg administered twice daily. However, the tested fluvoxamine dose was suboptimal. Full therapeutic dose of fluvoxamine is expected to cause a greater increase in asenapine exposure.
Figure 2: Effect of Other Drugs on Asenapine Pharmacokinetics
The effects of asenapine on the pharmacokinetics of other co-administered drugs are summarized in Figure 3.
Figure 3: Effect of Asenapine on Other Drug Pharmacokinetics
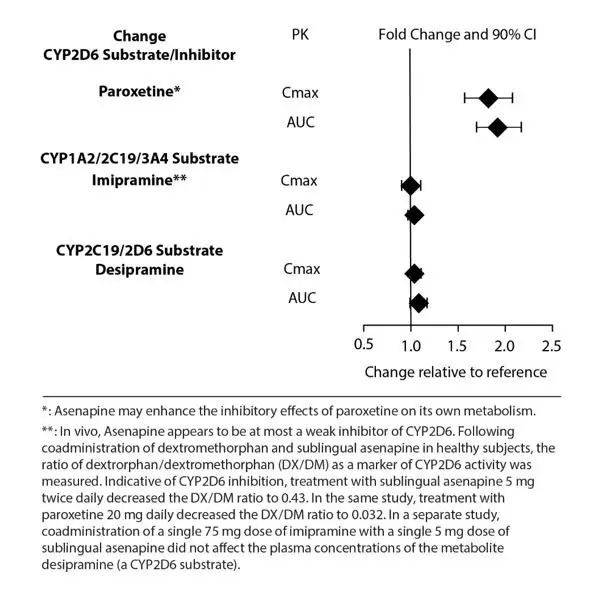
In vitro studies indicate that asenapine is a substrate for UGT1A4, CYP1A2 and to a lesser extent CYP3A4 and CYP2D6. Asenapine is a weak inhibitor of CYP2D6. Asenapine does not cause induction of CYP1A2 or CYP3A4 activities in cultured human hepatocytes. Coadministration of asenapine with known inhibitors, inducers or substrates of these metabolic pathways has been studied in a number of drug-drug interaction studies [see Drug Interactions (7.1)].
13. Nonclinical Toxicology
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis: In a lifetime carcinogenicity study in CD-1 mice asenapine was administered subcutaneously at doses up to those resulting in plasma levels (AUC) estimated to be 5 times those in humans receiving the MRHD of 10.0 mg twice daily. The incidence of malignant lymphomas was increased in female mice, with a no-effect dose resulting in plasma levels estimated to be 1.5 times those in humans receiving the MRHD. The mouse strain used has a high and variable incidence of malignant lymphomas, and the significance of these results to humans is unknown. There were no increases in other tumor types in female mice. In male mice, there were no increases in any tumor type.
In a lifetime carcinogenicity study in Sprague-Dawley rats, asenapine did not cause any increases in tumors when administered subcutaneously at doses up to those resulting in plasma levels (AUC) estimated to be 5 times those in humans receiving the MRHD.
In a 39-week study in minipigs, the asenapine transdermal system was administered at doses of 0.43 to 3.84 mg/kg asenapine, once every 24 hours. No significant dermal findings occurred at doses up to 17 times the MRHD of 12.8 mg transdermal asenapine daily for schizophrenia.
Mutagenesis: No evidence for genotoxic potential of asenapine was found in the in vitro bacterial reverse mutation assay, the in vitro forward gene mutation assay in mouse lymphoma cells, the in vitro chromosomal aberration assays in human lymphocytes, the in vitro sister chromatid exchange assay in rabbit lymphocytes, or the in vivo micronucleus assay in rats.
Impairment of Fertility: Asenapine did not impair fertility in rats when tested at doses up to 11 mg/kg twice daily given orally. This dose is 10 times MRHD of 10 mg twice daily asenapine given sublingually on a mg/m2 basis and 16.6 times the MRHD of 12.8 mg daily asenapine given transdermally on a mg/m2 basis.
13.2 Animal Toxicology and/or Pharmacology
Transdermal administration of asenapine to rats, dogs and minipigs did not demonstrate any significant dermal findings when applied daily for 24 hours. Rats were treated for 26-weeks with percutaneous doses of asenapine (as free base) up to 1.42 mg/kg (1.3 times the MRHD of 12.8 mg transdermal asenapine as free base daily on a mg/kg basis), dogs were treated for 13-weeks with percutaneous doses of asenapine (as free base) up to 5.6 mg/kg (14.2 times the MRHD of 12.8 mg transdermal asenapine daily on mg/m2 basis) and minipigs were treated for 39-weeks with percutaneous doses of asenapine (as free base) up to 3.84 mg/kg (17 times the MRHD of 12.8 mg transdermal asenapine [as free base] daily on mg/m2 basis).
14. Clinical Studies
The efficacy of SECUADO in the treatment of adult patients with schizophrenia was established, in part, on the basis of efficacy data from trials with the sublingual formulation of asenapine. In addition, the efficacy of SECUADO was evaluated in a 6-week, fixed-dose, randomized, double-blind, and placebo-controlled trial (Study 1; NCT 02876900) of adult patients who met DSM-IV criteria for schizophrenia.
In Study 1, the Positive and Negative Syndrome Scale (PANSS) and Clinical Global Impressions-Severity (CGI-S) rating scales were used as the primary and key secondary efficacy measures, respectively, for assessing psychiatric signs and symptoms in each trial:
- PANSS is a 30 item scale that measures positive symptoms of schizophrenia (7 items), negative symptoms of schizophrenia (7 items), and general psychopathology (16 items), each rated on a scale of 1 (absent) to 7 (extreme); total PANSS scores range from 30 to 210.
- CGI-S is a validated clinician-rated scale that measures the patient’s current illness state and overall clinical state on a 1 (normal, not at all ill) to 7-point (extremely ill) scale, based on the rater’s total clinical experience with this population.
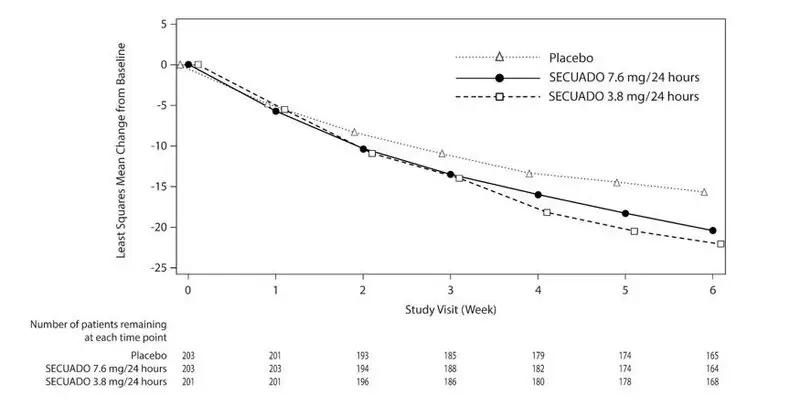
The primary endpoint was change from baseline in PANSS total score to Week 6. The change from baseline for SECUADO was compared to that for placebo. The results of the trial are shown in Table 8. The time course of efficacy is shown in Figure 4.
In the 6-week trial (n=607) comparing two fixed doses of SECUADO (3.8 mg/24 hours and 7.6 mg/24 hours) to placebo, both doses were statistically superior to placebo for both PANSS total score and CGI-S.
An examination of population subgroups did not reveal any clear evidence of differential responsiveness on the basis of age, sex or race.
|
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval.
|
|||
| Treatment Group | Primary Efficacy Measure: PANSS Total Score | ||
|---|---|---|---|
| Mean Baseline Score (SD) | LS Mean Change from Baseline (SE) to Week 6 | Placebo-subtracted Differencea (95% CI) | |
| SECUADO 3.8 mg/24 hours* | 97.0 (9.78) | -22.1 (1.2) | -6.6 (-9.81, -3.40) |
| SECUADO 7.6 mg/24 hours* | 95.6 (8.68) | -20.4 (1.2) | -4.8 (-8.06, -1.64) |
| Placebo | 97.4 (10.07) | -15.5 (1.2) | -- |
Maintenance of Efficacy with Sublingual Asenapine
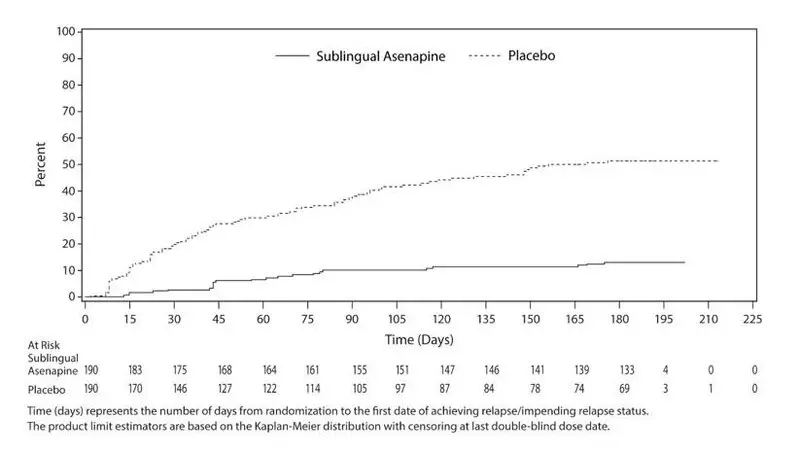
Maintenance of efficacy has been demonstrated in a placebo-controlled, double-blind, multicenter, flexible dose with sublingual asenapine (5 mg or 10 mg twice daily based on tolerability) clinical trial with a randomized withdrawal design. All patients were initially administered 5 mg twice daily for 1 week and then titrated up to 10 mg twice daily. A total of 700 patients entered open label treatment with sublingual asenapine for a period of 26 weeks. Of these, a total of 386 patients who met pre-specified criteria for continued stability (mean length of stabilization was 22 weeks) were randomized to a double-blind, placebo-controlled, randomized withdrawal phase. Sublingual asenapine was statistically superior to placebo in time to relapse or impending relapse defined as increase in PANSS ≥20% from baseline and a Clinical Global Impression–Severity of Illness (CGI-S) score ≥4 (at least 2 days within 1 week) or PANSS score ≥5 on "hostility" or "uncooperativeness" items and CGI-S score ≥4 (≥2 days within a week), or PANSS score ≥5 on any two of the following items: "unusual thought content," "conceptual disorganization," or "hallucinatory behavior" items, and CGI-S score ≥4 (≥2 days within 1 week) or investigator judgment of worsening symptoms or increased risk of violence to self (including suicide) or other persons.The Kaplan-Meier curves of the time to relapse or impending relapse during the randomized, double-blind, placebo-controlled withdrawal phase of this trial for asenapine and placebo are shown in Figure 5.
Figure 5: Kaplan-Meier Estimation of Percent Relapse for Sublingual Asenapine and Placebo
Adhesion
Based on a clinical study in 40 subjects, each wearing one SECUADO 3.8 mg/24 hours, 40 transdermal systems (100%) exhibited 75% or greater surface area adhesion at all timepoints evaluated (every 4 hours) throughout the 24-hour wear period. Based on a clinical study in 39 subjects, each wearing one SECUADO 7.6 mg/24 hours, 36 transdermal systems (92%) exhibited 75% or greater surface area adhesion at all timepoints evaluated (every 4 hours) throughout the 24-hour wear period. One SECUADO 7.6 mg/24 hours transdermal system worn on the hip fully detached.
16. How is Secuado Film supplied
16.1 How Supplied
SECUADO (asenapine) transdermal system is a translucent rounded square product with a printed backing on one side and a release liner on the other supplied as:
-
3.8 mg/24 hours transdermal system (system size: 20 cm2)
Carton of 30 transdermal systems, each transdermal system is packaged in an individual pouch
NDC 68968-0172-3 -
5.7 mg/24 hours transdermal system (system size: 30 cm2)
Carton of 30 systems, each system is packaged into an individual pouch
NDC 68968-0173-3 -
7.6 mg/24 hours transdermal system (system size: 40 cm2)
Carton of 30 transdermal systems, each transdermal system is packaged in an individual pouch
NDC 68968-0174-3
17. Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Instructions for Use).
Hypersensitivity Reactions
Counsel patients on the signs and symptoms of a serious allergic reaction (e.g., difficulty breathing, itching, swelling of the face, tongue or throat, feeling lightheaded etc.) and to seek immediate emergency assistance if they develop any of these signs and symptoms [see Contraindications (4),Warnings and Precautions (5.6)].
Neuroleptic Malignant Syndrome
Counsel patients about a potentially fatal adverse reaction referred to as NMS that has been reported in association with administration of antipsychotic drugs. Advise patients to contact a healthcare provider or report to the emergency room if they experience signs or symptoms of NMS including hyperpyrexia, muscle rigidity, altered mental status, and evidence of autonomic instability (irregular pulse or blood pressure, tachycardia, diaphoresis, and cardiac dysrhythmia) [see Warnings and Precautions (5.3)].
Tardive Dyskinesia
Counsel patients on the signs and symptoms of tardive dyskinesia and to contact their health care provider if these abnormal movements occur [see Warnings and Precautions (5.4)].
Metabolic Changes (Hyperglycemia and Diabetes Mellitus, Dyslipidemia, and Weight Gain)
Educate patients about the risk of metabolic changes, how to recognize symptoms of hyperglycemia (high blood sugar) and diabetes mellitus, and the need for specific monitoring, including blood glucose, lipids, and weight [see Warnings and Precautions (5.5)].
Orthostatic Hypotension
Counsel patients about the risk of orthostatic hypotension (symptoms include feeling dizzy or lightheaded upon standing) especially early in treatment, and at times of re-initiating treatment or increases in dose [see Warnings and Precautions (5.7)].
Leukopenia/Neutropenia
Advise patients with a pre-existing low WBC or a history of drug induced leukopenia/neutropenia they should have their CBC monitored while taking SECUADO [see Warnings and Precautions (5.9)].
Hyperprolactinemia
Counsel patients on the signs and symptoms of hyperprolactinemia and to contact their health care provider if these abnormalities occur [see Warnings and Precautions (5.11)].
Interference with Cognitive and Motor Performance
Caution patients about performing activities requiring mental alertness, such as operating hazardous machinery or operating a motor vehicle, until they are reasonably certain that SECUADO therapy does not affect them adversely [see Warnings and Precautions (5.13)].
Heat Exposure and Dehydration
Counsel patients regarding appropriate care in avoiding overheating and dehydration [see Warnings and Precautions (5.14)].
External Heat
Inform patients to avoid exposing SECUADO to external heat sources, such as hair dryers, heating pads, electric blankets, heated water beds, etc., [see Warnings and Precautions (5.16)].
Application Site Reactions
Inform patients that application site reactions, including erythema, pruritus, papules, discomfort, pain, edema or irritation, have been reported with use of SECUADO. Inform patients that increased skin irritation may occur if applied for a longer period than instructed or if the same application site is used repeatedly. Instruct patients to select a different application site each day to minimize skin reactions. Patients should monitor for these reactions while wearing or immediately after removal of SECUADO [see Warnings and Precautions (5.17)].
Concomitant Medications
Advise patients to inform their health care provider if they are taking, or plan to take, any prescription or over-the-counter medications since there is a potential for interactions [see Drug Interactions (7.1)].
Pregnancy
Advise patients that SECUADO may cause fetal harm as well as extrapyramidal and/or withdrawal symptoms in a neonate. Advise patients to notify their healthcare provider with a known or suspected pregnancy [see Use in Specific Populations (8.1)].
Pregnancy Registry
Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to SECUADO during pregnancy [see Use in Specific Populations (8.1)].
Manufactured by: Hisamitsu Pharmaceutical Co. Inc., 408, Tashirodaikan-machi, Tosu, Saga, Japan
Distributed by: Noven Therapeutics, LLC, Miami, Florida USA.
For more information, call 1-800-455-8070 or visit www.secuado.com
SECUADO is a registered trademark of Hisamitsu Pharmaceutical Co., Inc.
© 2019-2022 Hisamitsu Pharmaceutical Co., Inc. All rights reserved.
3O134A-0
Instructions for Use
SECUADO® (seh kue a’ doe)
(asenapine) transdermal system
Read this Instructions for Use before you start using the SECUADO transdermal system (patch) and each time you get a refill. There may be new information. This information does not take the place of talking to your healthcare provider about your medical condition or treatment.
Important Information:
- The SECUADO transdermal system (patch) is for use on the skin only (transdermal).
- Do not cut the pouch open until you are ready to apply the patch.
- You should apply only 1 SECUADO patch to 1 application site every 24 hours. The patch should only be worn for 24 hours. Do not wear the patch longer than 24 hours.
- Avoid bathing or swimming while wearing the patch. Swimming or bathing may cause the patch to fall off. You may shower.
- Avoid exposing the patch application site to direct heat sources such as hair dryers, heating pads, electric blankets, or heated water beds.
- If your skin feels irritated or feels like it is burning after you apply the patch, remove the patch and apply a new patch to a new application site.
Applying your SECUADO patch:
- Change (rotate) your patch application site every time you apply a new patch. Changing your application site every time you apply a patch will help to lessen your chances of developing skin irritation at the application site. Do not use the same application site 2 times in a row.
- The application site you choose should be clean, dry, and intact. Do not apply the patch on skin that has cuts, scrapes, burns, rashes, redness, or other skin problems.
- The application site you choose should be hairless or nearly hairless. If there is a lot of hair, use scissors to clip the hair as close to the skin as possible. Do not shave the application site.
- Do not apply the patch on skin that has oils, lotions, or powders on it.
- Do not apply the patch to areas of your skin where you wear tight clothing, such as waistbands, bras, or tank top straps.
| Step 1. | 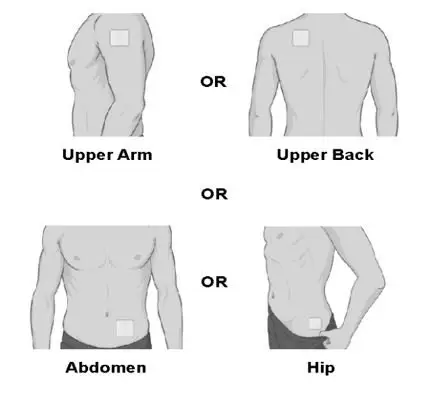 |
|
|
| Step 2.
When you are ready to apply your patch, use scissors and carefully cut the protective pouch along the dashed line to open the pouch and remove the patch. Do not cut the SECUADO patch. | 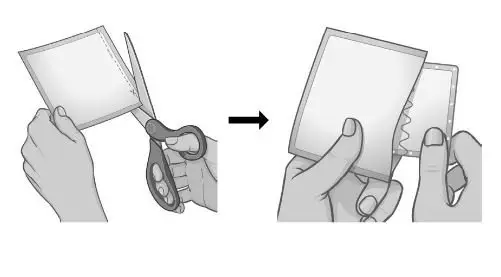 |
|
|
|
|
| Step 3.
Hold the patch with the protective liner facing you. Bend the patch along the wave-shaped line in the middle of the protective liner. Slowly peel half of the protective liner off your patch. | 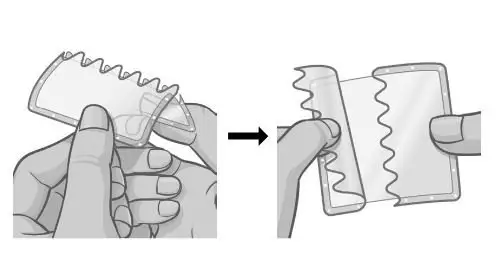 |
|
|
| Step 4.
Holding the other half of the protective liner, apply the sticky half of the patch to the application site you chose and smooth it down with your fingers. | 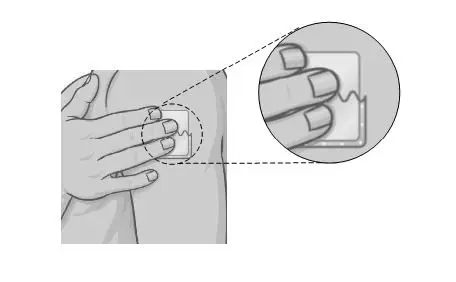 |
| Step 5.
Hold the edge of the remaining half of the protective liner, then slowly peel it away and smooth the patch down onto your skin with your fingers. | 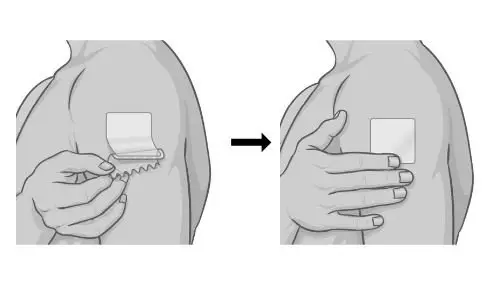 |
| Step 6.
Press and hold the patch firmly with the palm of your hand to make sure the patch and edges are sticking to your skin. |  |
|
|
|
|
|
|
| Step 7.
Wash your hands with soap and water after applying the patch. | 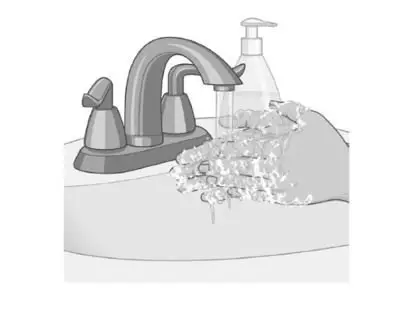 |
Removing and disposing of your used SECUADO patch:
| Step 8.
After you have worn the patch for 24 hours, remove the used patch from your skin and fold it in half so that the sticky sides stick together. | 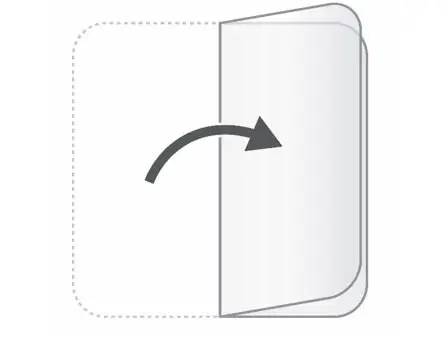 |
|
|
|
|
|
|
| Step 9.
Safely throw away the used folded patch in the trash right away so that children and pets cannot reach it | 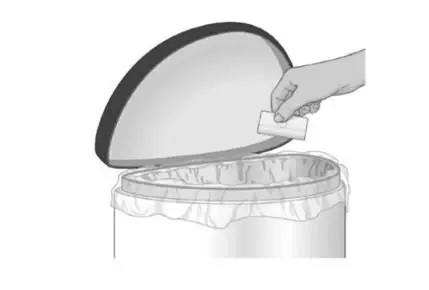 |
| Step 10.
Wash your hands with soap and water after removing the patch. | 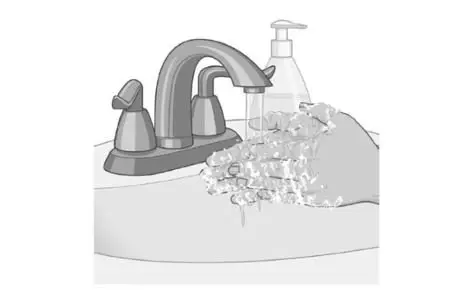 |
How should I store SECUADO patch?
- Store SECUADO patches at room temperature between 68°F to 77°F (20°C to 25°C).
- Keep SECUADO patches and all medicines out of the reach of children.
Manufactured by: Hisamitsu Pharmaceutical Co., Inc., 408, Tashirodaikan-machi, Tosu, Saga, Japan ©2019-2022, Hisamitsu Pharmaceutical Co., Inc. All rights reserved.
Distributed by: Noven Therapeutics, LLC, Miami, Florida USA
SECUADO is a registered trademark of Hisamitsu Pharmaceutical Co., Inc.
This Instructions for Use has been approved by the U.S. Food and Drug Administration
3O134A-0 Revised: 11/2022
| SECUADO
asenapine film, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| SECUADO
asenapine film, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| SECUADO
asenapine film, extended release |
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||
| Labeler - Noven Therapeutics, LLC (166888268) |